200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 26581-81-7
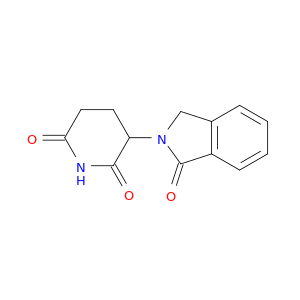
26581-81-7 | 2,6-Piperidinedione, 3-(1,3-dihydro-1-oxo-2H-isoindol-2-yl)-
CAS No: 26581-81-7 Catalog No: AG002TRV MDL No:MFCD01748359
Product Description
Catalog Number:
AG002TRV
Chemical Name:
2,6-Piperidinedione, 3-(1,3-dihydro-1-oxo-2H-isoindol-2-yl)-
CAS Number:
26581-81-7
Molecular Formula:
C13H12N2O3
Molecular Weight:
244.2460
MDL Number:
MFCD01748359
IUPAC Name:
3-(3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione
InChI:
InChI=1S/C13H12N2O3/c16-11-6-5-10(12(17)14-11)15-7-8-3-1-2-4-9(8)13(15)18/h1-4,10H,5-7H2,(H,14,16,17)
InChI Key:
WENKGSGGXGQHSH-UHFFFAOYSA-N
SMILES:
O=C1CCC(C(=O)N1)N1Cc2c(C1=O)cccc2
Properties
Complexity:
407
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
244.085g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
244.25g/mol
Monoisotopic Mass:
244.085g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
66.5A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
0.2
Literature
| Title | Journal |
|---|---|
| Comparative molecular field analysis and comparative molecular similarity indices analysis of thalidomide analogues as angiogenesis inhibitors. | Journal of medicinal chemistry 20040422 |
| Thalidomide metabolites and analogues. 3. Synthesis and antiangiogenic activity of the teratogenic and TNFalpha-modulatory thalidomide analogue 2-(2,6-dioxopiperidine-3-yl)phthalimidine. | Journal of medicinal chemistry 20030828 |
| Stimulation of gap junctional intercellular communication by thalidomide and thalidomide analogs in human fetal skin fibroblasts (HFFF2) and in rat liver epithelial cells (WB-F344). | Biochemical pharmacology 20011015 |
| The effect of thalidomide and 2 analogs on collagen induced arthritis. | The Journal of rheumatology 19980501 |
| Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. | Experimental eye research 19970601 |
| Down-regulation of adhesion receptors on cells of primate embryos as a probable mechanism of the teratogenic action of thalidomide. | Life sciences 19960101 |
| Embryotoxic effects of thalidomide derivatives in the non-human primate callithrix jacchus. IV. Teratogenicity of micrograms/kg doses of the EM12 enantiomers. | Teratogenesis, carcinogenesis, and mutagenesis 19940101 |
| The thalidomide analog, EM 12, enhances 1,2-dimethylhydrazine-induction of rat colon adenocarcinomas. | Cancer letters 19911101 |
| Embryotoxic effects of thalidomide derivatives on the non-human primate Callithrix jacchus; 3. Teratogenic potency of the EM 12 enantiomers. | Archives of toxicology 19880101 |
| Embryotoxic effects of thalidomide-derivatives in the non-human primate Callithrix jacchus. I. Effects of 3-(1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-dioxopiperidine (EM12) on skeletal development. | Archives of toxicology 19880101 |
| Platanna (Xenopus laevis) as a test organism for determining the embryotoxic effects of environmental chemicals. | Ecotoxicology and environmental safety 19840201 |
| The teratogenic activity of a thalidomide analogus EM12 in rats on a low-zinc diet. | Teratology 19790601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.