200,000+ products from a single source!
sales@angenechem.com
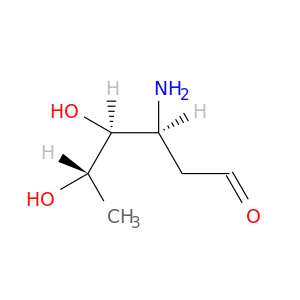
26548-47-0 | L-lyxo-Hexose, 3-amino-2,3,6-trideoxy-
CAS No: 26548-47-0 Catalog No: AG002TNL MDL No:
Product Description
Catalog Number:
AG002TNL
Chemical Name:
L-lyxo-Hexose, 3-amino-2,3,6-trideoxy-
CAS Number:
26548-47-0
Molecular Formula:
C6H13NO3
Molecular Weight:
147.1723
IUPAC Name:
(3S,4S,5S)-3-amino-4,5-dihydroxyhexanal
InChI:
InChI=1S/C6H13NO3/c1-4(9)6(10)5(7)2-3-8/h3-6,9-10H,2,7H2,1H3/t4-,5-,6+/m0/s1
InChI Key:
WPJRFCZKZXBUNI-HCWXCVPCSA-N
SMILES:
C[C@@H]([C@H]([C@H](CC=O)N)O)O
Properties
Complexity:
107
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
147.09g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
147.174g/mol
Monoisotopic Mass:
147.09g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
83.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.1
Literature
| Title | Journal |
|---|---|
| Oxazolinodoxorubicin - a promising new anthracycline. | Anticancer research 20120701 |
| Synthesis and biological activities of a 3'-azido analogue of Doxorubicin against drug-resistant cancer cells. | International journal of molecular sciences 20120101 |
| DNA interactions of 2-pyrrolinodoxorubicin, a distinctively more potent daunosamine-modified analogue of doxorubicin. | Biochemical pharmacology 20110801 |
| A short and highly efficient synthesis of L-ristosamine and L-epi-daunosamine glycosides. | Organic letters 20110218 |
| Synthesis and biological properties of oxazolinodaunorubicin--a new derivative of daunorubicin with a modified daunosamine moiety. | Investigational new drugs 20101001 |
| Limitations in doxorubicin production from Streptomyces peucetius. | Microbiological research 20100720 |
| Overexpression of aveBIV leading to the improvement of 4'-epidaunorubicin production in Streptomyces coeruleorubidus strain SIPI-A0707. | Applied microbiology and biotechnology 20100701 |
| Precursor for biosynthesis of sugar moiety of doxorubicin depends on rhamnose biosynthetic pathway in Streptomyces peucetius ATCC 27952. | Applied microbiology and biotechnology 20100201 |
| Synthesis and DNA-binding affinity studies of glycosylated intercalators designed as functional mimics of the anthracycline antibiotics. | Organic & biomolecular chemistry 20090921 |
| Amidinoanthracyclines - a new group of potential anti-hepatitis C virus compounds. | Biological chemistry 20090401 |
| An in silico approach to map the binding site of doxorubicin on hemoglobin. | Bioinformation 20080101 |
| Next-generation anthracycline for the management of small cell lung cancer: focus on amrubicin. | Drug design, development and therapy 20080101 |
| Synthesis of daunorubicin analogues containing truncated aromatic cores and unnatural monosaccharide residues. | The Journal of organic chemistry 20070413 |
| Aldehyde-selective Wacker oxidation in a thiyl-mediated vinyl group transfer route to daunosamine. | Organic letters 20070301 |
| Biological properties of new derivatives of daunorubicin. | In vivo (Athens, Greece) 20070101 |
| Synthesis of L-daunosamine and L-ristosamine glycosides via photoinduced aziridination. Conversion to thioglycosides for use in glycosylation reactions. | The Journal of organic chemistry 20061013 |
| The Ramberg-Bäcklund reaction for the synthesis of C-glycosides, C-linked-disaccharides and related compounds. | Carbohydrate research 20060724 |
| A stereoselective synthesis of 6,6,6-trifluoro-L-daunosamine and 6,6,6-trifluoro-L-acosamine. | Organic & biomolecular chemistry 20060721 |
| Effect of structural modifications of anthracyclines on the ability to overcome drug resistance of cancer cells. | Anticancer research 20060101 |
| A new bisintercalating anthracycline with picomolar DNA binding affinity. | Journal of medicinal chemistry 20051229 |
| Effects of anthracycline derivatives on human leukemia K562 cell growth and differentiation. | Biochemical pharmacology 20051115 |
| Improved synthesis of daunomycin conjugates with triplex-forming oligonucleotides. The polypurine tract of HIV-1 as a target. | Bioorganic & medicinal chemistry 20050502 |
| Regioselectivity of rhodium nitrene insertion. Syntheses of protected glycals of l-daunosamine, d-saccharosamine, and l-ristosamine. | Organic letters 20050428 |
| Sequence specificity of formaldehyde-mediated covalent binding of anthracycline derivatives to DNA. | Biochemical pharmacology 20050101 |
| Cytotoxicity, cellular uptake and DNA damage by daunorubicin and its new analogues with modified daunosamine moiety. | Cell biology and toxicology 20050101 |
| AknK is an L-2-deoxyfucosyltransferase in the biosynthesis of the anthracycline aclacinomycin A. | Biochemistry 20040420 |
| Interactions of novel morpholine and hexamethylene derivatives of anthracycline antibiotics with DNA. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20040101 |
| New stereodivergent approach to 3-amino-2,3,6-trideoxysugars. Enantioselective synthesis of daunosamine, ristosamine, acosamine, and epi-daunosamine. | Organic letters 20030821 |
| Structure of the first parallel DNA quadruplex-drug complex. | Journal of the American Chemical Society 20030409 |
| Inhibition of RNA synthesis in vitro and cell growth by anthracycline antibiotics. | Neoplasma 20010101 |
| A two-plasmid system for the glycosylation of polyketide antibiotics: bioconversion of epsilon-rhodomycinone to rhodomycin D. | Chemistry & biology 19991201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.








