200,000+ products from a single source!
sales@angenechem.com
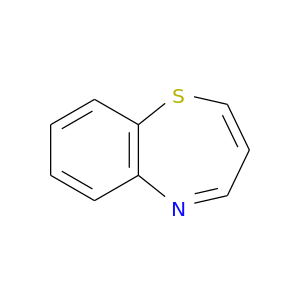
265-13-4 | 1,5-Benzothiazepine
CAS No: 265-13-4 Catalog No: AG002THC MDL No:
Product Description
Catalog Number:
AG002THC
Chemical Name:
1,5-Benzothiazepine
CAS Number:
265-13-4
Molecular Formula:
C9H7NS
Molecular Weight:
161.2236
IUPAC Name:
1,5-benzothiazepine
InChI:
InChI=1S/C9H7NS/c1-2-5-9-8(4-1)10-6-3-7-11-9/h1-7H
InChI Key:
KJFRSZASZNLCDF-UHFFFAOYSA-N
SMILES:
C1=CSc2c(N=C1)cccc2
Properties
Complexity:
186
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
161.03g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
161.222g/mol
Monoisotopic Mass:
161.03g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
37.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Synthesis of 1,5-benzothiazepine derivatives bearing 2-phenoxy-quinoline moiety via 1,3-diplolar cycloaddition reaction. | Molecular diversity 20111101 |
| Modulation of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) by 6-arylpyrrolo[2,1-d][1,5]benzothiazepine derivatives, ligands of peripheral benzodiazepine receptor (PBR). | Toxicology letters 20110425 |
| Comparative Effects of α-, β-, and γ-Carbolines on Platelet Aggregation and Lipid Membranes. | Journal of toxicology 20110101 |
| Three-component synthesis of benzo[b][1,5]thiazepines via coupling-addition-cyclocondensation sequence. | Molecular diversity 20100801 |
| Synthesis of 1,5-benzothiazepine dipeptide mimetics via two CuI-catalyzed cross coupling reactions. | Organic letters 20090702 |
| Synthesis and biological evaluation of a novel series of 1,5-benzothiazepine derivatives as potential antimicrobial agents. | European journal of medicinal chemistry 20090701 |
| Pharmacological characteristics and clinical applications of K201. | Current clinical pharmacology 20090501 |
| Design, synthesis, and biological evaluation of 1,5-benzothiazepine-4-one derivatives targeting factor VIIa/tissue factor. | Bioorganic & medicinal chemistry letters 20090301 |
| 1,5-Benzothiazepine, a versatile pharmacophore: a review. | European journal of medicinal chemistry 20081101 |
| Solid-phase synthesis and biological evaluation of a parallel library of 2,3-dihydro-1,5-benzothiazepines. | Bioorganic & medicinal chemistry 20080815 |
| Mitochondrial Ca2+ homeostasis in human NADH:ubiquinone oxidoreductase deficiency. | Cell calcium 20080701 |
| Molecular modeling of benzothiazepine binding in the L-type calcium channel. | The Journal of biological chemistry 20080620 |
| Regio- and stereocontrolled synthesis of novel 3-sulfonamido-2,3,4,5-tetrahydro-1,5-benzothiazepines from 2-(bromomethyl)- or 2-(sulfonyloxymethyl)aziridines. | Organic & biomolecular chemistry 20080607 |
| Synthesis and structure characterization of new 1,2,4-oxadiazolo[4,5-d]-1,5-benzothiazepines derivatives containing 2-phenyl-1,2,3-triazole through 1,3-dipolar cycloaddition reaction. | Molecular diversity 20080501 |
| Structural insight into the inhibition of acetylcholinesterase by 2,3,4, 5-tetrahydro-1, 5-benzothiazepines. | Journal of enzyme inhibition and medicinal chemistry 20080401 |
| The expedient synthesis of 1,5-benzothiazepines as a family of cytotoxic drugs. | Bioorganic & medicinal chemistry letters 20080101 |
| Syntheses and biological activities of chalcone and 1,5-benzothiazepine derivatives: promising new free-radical scavengers, and esterase, urease, and alpha-glucosidase inhibitors. | Chemistry & biodiversity 20050401 |
| A combinatorial approach to [1,5]benzothiazepine derivates as potential antibacterial agents. | Journal of combinatorial chemistry 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







