200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 265-02-1
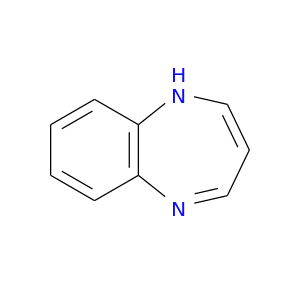
265-02-1 | 1H-1,5-Benzodiazepine
CAS No: 265-02-1 Catalog No: AG002THE MDL No:
Product Description
Catalog Number:
AG002THE
Chemical Name:
1H-1,5-Benzodiazepine
CAS Number:
265-02-1
Molecular Formula:
C9H8N2
Molecular Weight:
144.1732
IUPAC Name:
1H-1,5-benzodiazepine
InChI:
InChI=1S/C9H8N2/c1-2-5-9-8(4-1)10-6-3-7-11-9/h1-7,10H
InChI Key:
ZVAPWJGRRUHKGP-UHFFFAOYSA-N
SMILES:
C1=CNc2c(N=C1)cccc2
Properties
Complexity:
184
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
144.069g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
144.177g/mol
Monoisotopic Mass:
144.069g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
24.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. | CNS drugs 20120301 |
| Synthesis of 1,5-benzodiazepine and its derivatives by condensation reaction using H-MCM-22 as catalyst. | Journal of biomedicine & biotechnology 20120101 |
| Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. | Neurology 20111011 |
| Toxicological approach for elucidation of clobazam-induced hepatomegaly in male rats. | Regulatory toxicology and pharmacology : RTP 20110801 |
| Ethyl 5,5-dichloro-3-(4-chloro-phen-yl)-3a-methyl-4a-phenyl-3a,4,4a,5-tetra-hydro-3H-aziridino[2,1-d][1,2,4]triazolo[4,3-a][1,5]benzodiazepine-1-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20110501 |
| Racemic 9,10-dimeth-oxy-3-methyl-6-phenyl-7,7a-dihydro-benzo[b]benzo[4,5]isothia-zolo[2,3-d][1,4]diazepine 12,12-dioxide. | Acta crystallographica. Section E, Structure reports online 20110301 |
| [Effect of 1,5-benzodiazepine derivatives on the electrical activity of neurons of the Helix albescens Rossm]. | Fiziolohichnyi zhurnal (Kiev, Ukraine : 1994) 20110101 |
| Clobazam as an adjunctive therapy in treating seizures associated with Lennox-Gastaut syndrome. | Neuropsychiatric disease and treatment 20110101 |
| Synthesis of substituted 1,4-diazepines and 1,5-benzodiazepines using an efficient heteropolyacid-catalyzed procedure. | Molecules (Basel, Switzerland) 20101228 |
| Anti-neuroinflammatory activity of 1,5-benzodiazepine derivatives. | Bioorganic & medicinal chemistry letters 20100701 |
| Cytogenetic activity of newly synthesized 1,5-benzodiazepines in normal human lymphocyte cultures. | Genetic testing and molecular biomarkers 20100601 |
| New 1,5-benzodiazepine compounds: activity at native GABA(A) receptors. | Neuroscience 20100331 |
| Neuropharmacological screening of two 1,5-benzodiazepine compounds in mice. | Comptes rendus biologies 20100301 |
| Sodium tetrachloroaurate(III) dihydrate-catalyzed efficient synthesis of 1,5-benzodiazepine and quinoxaline derivatives. | Journal of Zhejiang University. Science. B 20100201 |
| Diethyl 1,4-bis-(4-nitro-phen-yl)-1,4-dihydro-1,2,4,5-tetra-zine-3,6-dicarboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20100201 |
| [Therapeutic drug monitoring of clobazam]. | Therapie 20100101 |
| Z-360, a novel therapeutic agent for pancreatic cancer, prevents up-regulation of ephrin B1 gene expression and phosphorylation of NR2B via suppression of interleukin-1 β production in a cancer-induced pain model in mice. | Molecular pain 20100101 |
| Enaminonitrile in heterocyclic synthesis: synthesis and antimicrobial evaluation of some new pyrazole, isoxazole and pyrimidine derivatives incorporating a benzothiazole moiety. | European journal of medicinal chemistry 20091201 |
| 2-Methyl-2,4-di-4-pyridyl-2,3-dihydro-1H-1,5-benzodiazepine acetic acid solvate. | Acta crystallographica. Section E, Structure reports online 20091201 |
| 1,5-Benzodiazepine inhibitors of HCV NS5B polymerase. | Bioorganic & medicinal chemistry letters 20090501 |
| Treatment of Lennox-Gastaut syndrome: overview and recent findings. | Neuropsychiatric disease and treatment 20081201 |
| Efficient TCT-catalyzed synthesis of 1,5-benzodiazepine derivatives under mild conditions. | Molecules (Basel, Switzerland) 20080925 |
| Gas-phase fragmentation study of novel synthetic 1,5-benzodiazepine derivatives using electrospray ionization tandem mass spectrometry. | Rapid communications in mass spectrometry : RCM 20080701 |
| 4-(2-Hydroxyphenyl)-2-phenyl-2,3-dihydro-1H-1,5-benzodiazepine and the 2-(2,3-dimethoxyphenyl)-, 2-(3,4-dimethoxyphenyl)- and 2-(2,5-dimethoxyphenyl)-substituted derivatives. | Acta crystallographica. Section C, Crystal structure communications 20070701 |
| New inhibitors of fungal 17beta-hydroxysteroid dehydrogenase based on the [1,5]-benzodiazepine scaffold. | Journal of enzyme inhibition and medicinal chemistry 20070201 |
| Strategies for design of non peptide CCK1R agonist/antagonist ligands. | Current topics in medicinal chemistry 20070101 |
| Transition metal complexes of quinolino[3,2-b]benzodiazepine and quinolino[3,2-b]benzoxazepine: synthesis, characterization, and antimicrobial studies. | Bioinorganic chemistry and applications 20070101 |
| In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. | Drug metabolism and disposition: the biological fate of chemicals 20060401 |
| Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. | Journal of medicinal chemistry 20060209 |
| 1H and 13C NMR identification of unexpected 3,4-dihydroquinoxalines in the syntheses of 1,5-benzodiazepine derivatives. | Magnetic resonance in chemistry : MRC 20050701 |
| Mechanism of clobazam-induced thyroidal oncogenesis in male rats. | Toxicology letters 20031210 |
| [A pharmacological profile of clobazam (Mystan), a new antiepileptic drug]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20010801 |
| 1,5-Benzodiazepine tricyclic derivatives exerting anti-inflammatory effects in mice by inhibiting interleukin-6 and prostaglandinE(2)production. | Pharmacological research 20010501 |
| Biologically active substituted benzodiazepines and their effect on cardiovascular and central nervous system. | Bollettino chimico farmaceutico 20010101 |
| 1,5-Benzodiazepines. Part XII. Synthesis and biological evaluation of tricyclic and tetracyclic 1,5-benzodiazepine derivatives as nevirapine analogues. | European journal of medicinal chemistry 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







