200,000+ products from a single source!
sales@angenechem.com
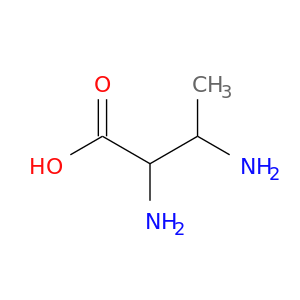
2643-66-5 | Butanoic acid, 2,3-diamino-
CAS No: 2643-66-5 Catalog No: AG002T6Y MDL No:MFCD09836087
Product Description
Catalog Number:
AG002T6Y
Chemical Name:
Butanoic acid, 2,3-diamino-
CAS Number:
2643-66-5
Molecular Formula:
C4H10N2O2
Molecular Weight:
118.1344
MDL Number:
MFCD09836087
IUPAC Name:
2,3-diaminobutanoic acid
InChI:
InChI=1S/C4H10N2O2/c1-2(5)3(6)4(7)8/h2-3H,5-6H2,1H3,(H,7,8)
InChI Key:
SXGMVGOVILIERA-UHFFFAOYSA-N
SMILES:
CC(C(C(=O)O)N)N
Properties
Complexity:
94
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
118.074g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
118.136g/mol
Monoisotopic Mass:
118.074g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
89.3A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
-3.2
Literature
| Title | Journal |
|---|---|
| Natural products from the Lithistida: a review of the literature since 2000. | Marine drugs 20111201 |
| Discovery of α,β- and α,γ-diamino acid scaffolds for the inhibition of M1 family aminopeptidases. | ChemMedChem 20111104 |
| Mutation of L-2,3-diaminopropionic acid synthase genes blocks staphyloferrin B synthesis in Staphylococcus aureus. | BMC microbiology 20110101 |
| Aspartocin cyclic lipopeptide antibiotics: mass spectral structural confirmations and the diagnostic role played by the alpha,beta-diaminobutyric acid residue. | Journal of mass spectrometry : JMS 20100701 |
| Analytical protocol for identification of BMAA and DAB in biological samples. | The Analyst 20100101 |
| Cyclodepsipeptides from marine sponges: natural agents for drug research. | Marine drugs 20100101 |
| Predicted class-I aminoacyl tRNA synthetase-like proteins in non-ribosomal peptide synthesis. | Biology direct 20100101 |
| Antiviral lead compounds from marine sponges. | Marine drugs 20100101 |
| Potential anti-HIV agents from marine resources: an overview. | Marine drugs 20100101 |
| Structure characterization of lipocyclopeptide antibiotics, aspartocins A, B & C, by ESI-MSMS and ESI-nozzle-skimmer-MSMS. | Journal of mass spectrometry : JMS 20091201 |
| Characterizing the anti-HIV activity of papuamide A. | Marine drugs 20081201 |
| Identification of a novel beta-replacement reaction in the biosynthesis of 2,3-diaminobutyric acid in peptidylnucleoside mureidomycin A. | Organic & biomolecular chemistry 20080607 |
| Stereocontrolled route to vicinal diamines by [3.3] sigmatropic rearrangement of allyl cyanate: asymmetric synthesis of anti-(2R,3R)- and syn-(2R,3S)-2,3-diaminobutanoic acids. | Organic letters 20061207 |
| Expedient asymmetric synthesis of all four isomers of N,N'-protected 2,3-diaminobutanoic acid. | The Journal of organic chemistry 20010615 |
Related Products
© 2019 Angene International Limited. All rights Reserved.