200,000+ products from a single source!
sales@angenechem.com
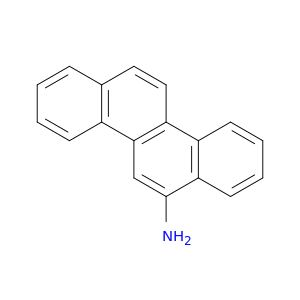
2642-98-0 | 6-Chrysenamine
CAS No: 2642-98-0 Catalog No: AG002T4Y MDL No:
Product Description
Catalog Number:
AG002T4Y
Chemical Name:
6-Chrysenamine
CAS Number:
2642-98-0
Molecular Formula:
C18H13N
Molecular Weight:
243.3025
IUPAC Name:
chrysen-6-amine
InChI:
InChI=1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
InChI Key:
KIVUHCNVDWYUNP-UHFFFAOYSA-N
SMILES:
Nc1cc2c(c3c1cccc3)ccc1c2cccc1
EC Number:
220-149-7
UNII:
I56L81BL2L
NSC Number:
80186
Properties
Complexity:
326
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
243.105g/mol
Formal Charge:
0
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
243.309g/mol
Monoisotopic Mass:
243.105g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
26A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5
Literature
| Title | Journal |
|---|---|
| Comparing cytotoxicity and genotoxicity in HaCaT cells caused by 6-aminochrysene and 5,6-chrysenequinone under ultraviolet A irradiation. | Environmental toxicology and chemistry 20060701 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6 and 2E1: a new cell expression system to study cytochrome P450 mediated biotransformation. | Mutagenesis 20050301 |
| Black tea intake modulates the excretion of urinary mutagens in rats exposed to 6-aminochrysene: induction of cytochromes P450 by 6-aminochrysene in the rat. | Mutagenesis 20050101 |
| Identification of 6-aminochrysene photoproducts and study of the effect of a humic acid and riboflavin on its photolysis. | Journal of photochemistry and photobiology. B, Biology 20031205 |
| Analysis of 6-(2,2-Dichloroacetamido)chrysene interaction with the hypoxanthine phosphoribosyltransferase from Trypanosoma cruzi. | Journal of medicinal chemistry 20030605 |
| Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. | Carcinogenesis 20020701 |
| Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: chemical-, cytochrome P450 isoform-, and cell-specific differences. | Archives of toxicology 20020601 |
| Mutagenicity of bay-region amino-substituted cyclopenta[a]phenanthrenes and 2- and 5-aminochrysene. | Mutation research 20010531 |
| Polycyclic aromatic compounds as anticancer agents: structure-activity relationships of chrysene and pyrene derivatives. | Bioorganic & medicinal chemistry 20010301 |
| Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in Saccharomyces cerevisiae. | Biochemistry 19901225 |
| Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. | Cancer research 19890615 |
| Structure and function of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Species difference in molecular properties of the receptors from mouse and rat hepatic cytosols. | The Journal of biological chemistry 19860325 |
| Effects of cytochrome P1-450 inducers on the cell-surface receptors for epidermal growth factor, phorbol 12,13-dibutyrate, or insulin of cultured mouse hepatoma cells. | The Journal of biological chemistry 19830910 |
Related Products
© 2019 Angene International Limited. All rights Reserved.