200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 26314-10-3
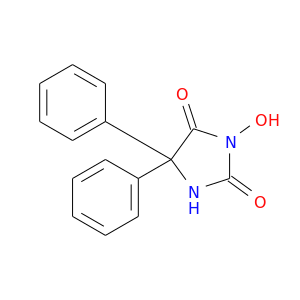
26314-10-3 | 2,4-Imidazolidinedione, 3-hydroxy-5,5-diphenyl-
CAS No: 26314-10-3 Catalog No: AG002SOV MDL No:
Product Description
Catalog Number:
AG002SOV
Chemical Name:
2,4-Imidazolidinedione, 3-hydroxy-5,5-diphenyl-
CAS Number:
26314-10-3
Molecular Formula:
C15H12N2O3
Molecular Weight:
268.2674
IUPAC Name:
3-hydroxy-5,5-diphenylimidazolidine-2,4-dione
InChI:
InChI=1S/C15H12N2O3/c18-13-15(16-14(19)17(13)20,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,20H,(H,16,19)
InChI Key:
RJSPYBADHBTXNB-UHFFFAOYSA-N
SMILES:
ON1C(=O)NC(C1=O)(c1ccccc1)c1ccccc1
Properties
Complexity:
380
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
268.085g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
268.272g/mol
Monoisotopic Mass:
268.085g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
69.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.8
Literature
| Title | Journal |
|---|---|
| CYP2C9 amino acid residues influencing phenytoin turnover and metabolite regio- and stereochemistry. | The Journal of pharmacology and experimental therapeutics 20090601 |
| Assessment of urinary mephenytoin metrics to phenotype for CYP2C19 and CYP2B6 activity. | European journal of clinical pharmacology 20080401 |
| Pharmacokinetics of phenytoin and its metabolite, 4'-HPPH, after intravenous and oral administration of phenytoin to diabetic rats induced by alloxan or streptozotocin. | Biopharmaceutics & drug disposition 20080101 |
| CYP2C9 inhibition: impact of probe selection and pharmacogenetics on in vitro inhibition profiles. | Drug metabolism and disposition: the biological fate of chemicals 20061201 |
| Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19*1/*2 patients. | Epilepsy research 20060901 |
| CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. | Pharmacogenetics and genomics 20051101 |
| Urinary excretion of phenytoin metabolites, 5-(4'-hydroxyphenyl)-5-phenylhydantoin and its O-glucuronide in humans and analysis of genetic polymorphisms of UDP-glucuronosyltransferases. | Drug metabolism and pharmacokinetics 20050401 |
| The effects of phenytoin and its metabolite 5-(4-hydroxyphenyl)-5-phenylhydantoin on cellular glucose transport. | Life sciences 20050304 |
| Phenytoin overview--metabolite interference in some immunoassays could be clinically important. | Archives of pathology & laboratory medicine 20040701 |
| The hepatic and intestinal metabolic activities of P450 in rats with surgery- and drug-induced renal dysfunction. | Pharmaceutical research 20031001 |
| Involvement of multiple UDP-glucuronosyltransferase 1A isoforms in glucuronidation of 5-(4'-hydroxyphenyl)-5-phenylhydantoin in human liver microsomes. | Drug metabolism and disposition: the biological fate of chemicals 20021101 |
| Modeling and kinetic analysis of the reaction system using whole cells with separately and co-expressed D-hydantoinase and N-carbamoylase. | Biotechnology and bioengineering 20020630 |
| Identification of catalase in human livers as a factor that enhances phenytoin dihydroxy metabolite formation by human liver microsomes. | Biochemical pharmacology 20020615 |
| Effect of albumin on phenytoin and tolbutamide metabolism in human liver microsomes: an impact more than protein binding. | Drug metabolism and disposition: the biological fate of chemicals 20020601 |
| Phenytoin metabolic ratio: a putative marker of CYP2C9 activity in vivo. | Pharmacogenetics 20011001 |
| Stereoselective determination of p-hydroxyphenyl-phenylhydantoin enantiomers in rat liver microsomal incubates by reversed-phase high-performance liquid chromatography using beta-cyclodextrin as chiral mobile phase additives. | Biomedical chromatography : BMC 20010401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.