200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 26264-03-9
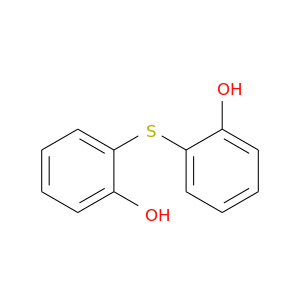
26264-03-9 | Phenol, thiobis-
CAS No: 26264-03-9 Catalog No: AG002SIU MDL No:
Product Description
Catalog Number:
AG002SIU
Chemical Name:
Phenol, thiobis-
CAS Number:
26264-03-9
Molecular Formula:
C12H10O2S
Molecular Weight:
218.2716
IUPAC Name:
2-(2-hydroxyphenyl)sulfanylphenol
InChI:
InChI=1S/C12H10O2S/c13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)14/h1-8,13-14H
InChI Key:
BLDLRWQLBOJPEB-UHFFFAOYSA-N
SMILES:
Oc1ccccc1Sc1ccccc1O
NSC Number:
522657
Properties
Complexity:
178
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
218.04g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
218.27g/mol
Monoisotopic Mass:
218.04g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
65.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.3
Literature
| Title | Journal |
|---|---|
| A convenient biomimetic synthesis of optically active putative neurotoxic metabolites of MDMA ('ecstasy') from R-(-)- and S-(+)-N-methyl-α-methyldopamine precursors. | Organic & biomolecular chemistry 20120514 |
| On-line electrochemistry-bioaffinity screening with parallel HR-LC-MS for the generation and characterization of modified p38α kinase inhibitors. | Analytical and bioanalytical chemistry 20120401 |
| Inhibition of 3,4-methylenedioxymethamphetamine metabolism leads to marked decrease in 3,4-dihydroxymethamphetamine formation but no change in serotonin neurotoxicity: implications for mechanisms of neurotoxicity. | Synapse (New York, N.Y.) 20111001 |
| Catecholthioether derivatives: preliminary study of in-vitro antimicrobial and antioxidant activities. | Chemical & pharmaceutical bulletin 20110101 |
| [Metabolites of ecstasy and cytotoxicity effects]. | Annales pharmaceutiques francaises 20090301 |
| Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy'): synthesis, isolation, and characterization of diastereoisomers. | Chemical research in toxicology 20081201 |
| Characterization of catechol-thioether-induced apoptosis in human SH-SY5Y neuroblastoma cells. | Journal of neuroscience research 20080301 |
| Bacterial plate assays and electrochemical methods: an efficient tandem for evaluating the ability of catechol-thioether metabolites of MDMA ('ecstasy') to induce toxic effects through redox-cycling. | Chemical research in toxicology 20070401 |
| 5-S-Cysteinyl-dopamine effect on the human dopaminergic neuroblastoma cell line SH-SY5Y. | Neurochemistry international 20060801 |
| Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions. | Water science and technology : a journal of the International Association on Water Pollution Research 20060101 |
| Thioether metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. | The Journal of pharmacology and experimental therapeutics 20041001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.