200,000+ products from a single source!
sales@angenechem.com
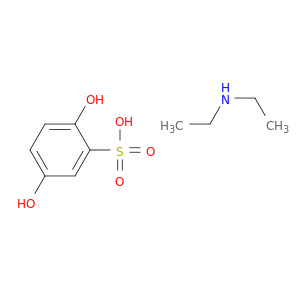
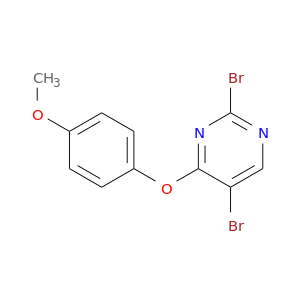
2624-44-4 | Benzenesulfonic acid, 2,5-dihydroxy-, compd. with N-ethylethanamine (1:1)
CAS No: 2624-44-4 Catalog No: AG002SF0 MDL No:MFCD00867499
Product Description
Catalog Number:
AG002SF0
Chemical Name:
Benzenesulfonic acid, 2,5-dihydroxy-, compd. with N-ethylethanamine (1:1)
CAS Number:
2624-44-4
Molecular Formula:
C10H17NO5S
Molecular Weight:
263.3107
MDL Number:
MFCD00867499
SMILES:
Oc1ccc(c(c1)S(=O)(=O)O)O.CCNCC
Literature
| Title | Journal |
|---|---|
| Suppression of bladder overactivity and oxidative stress by the phytotherapeutic agent, Eviprostat, in a rat model of atherosclerosis-induced chronic bladder ischemia. | International journal of urology : official journal of the Japanese Urological Association 20120701 |
| Short-term efficacy of intravitreal dobesilate in central serous chorioretinopathy. | European journal of medical research 20120101 |
| Intraventricular hemorrhage in preterm infants: coagulation perspectives. | Seminars in thrombosis and hemostasis 20111001 |
| Conventional and first derivative synchronous fluorometric determination of ethamsylate in pharmaceutical preparations and biological fluids. Application to stability studies. | Journal of fluorescence 20110701 |
| Effect of the phytotherapeutic agent Eviprostat on inflammatory changes and cytokine production in a rat model of nonbacterial prostatitis. | Urology 20110601 |
| Effect of a phytotherapeutic agent, Eviprostat®, on prostatic and urinary cytokines/chemokines in a rat model of nonbacterial prostatitis. | The Prostate 20110301 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Simultaneous determination of aminomethylbenzoic acid, cefminox sodium and etamsylate in human urine by capillary electrophoresis. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20100701 |
| A novel visible spectrophotometric method for the determination of ethamsylate in pharmaceutical preparations and biological samples. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20100301 |
| [Preparation and hemostatic evaluation of chitosan composite hemostatic membrane]. | Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery 20100301 |
| Eviprostat suppresses urinary oxidative stress in a rabbit model of partial bladder outlet obstruction and in patients with benign prostatic hyperplasia. | Phytotherapy research : PTR 20100201 |
| Ethamsylate for the prevention of morbidity and mortality in preterm or very low birth weight infants. | The Cochrane database of systematic reviews 20100120 |
| Application and equivalence assessment for determining ethamsylate by using potassium ferricyanide as spectroscopic probe reagent. | Analytical sciences : the international journal of the Japan Society for Analytical Chemistry 20100101 |
| Effect of the phytotherapeutic agent Eviprostat on 17beta-estradiol-induced nonbacterial inflammation in the rat prostate. | The Prostate 20090915 |
| Eviprostat suppresses proinflammatory gene expression in the prostate of rats with partial bladder-outlet obstruction: a genome-wide DNA microarray analysis. | Cytokine 20090901 |
| Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. | The Journal of urology 20090701 |
| [One-week effects of Tamsulosin on benign prostatic hyperplasia assessed with a daily symptom score]. | Hinyokika kiyo. Acta urologica Japonica 20090401 |
| [Assessment of clinical usefulness of Eviprostat for benign prostatic hyperplasia--comparison of Eviprostat tablet with a formulation containing two-times more active ingredients]. | Hinyokika kiyo. Acta urologica Japonica 20080601 |
| [Evaluation of supplemental administration of Eviprostat in patients with benign prostatic hyperplasia with persistent symptoms following treatment with alpha1-adrenoceptor blocker]. | Hinyokika kiyo. Acta urologica Japonica 20080501 |
| Effect of eviprostat on bladder overactivity in an experimental cystitis rat model. | International journal of urology : official journal of the Japanese Urological Association 20080401 |
| Mechanisms by which a phytotherapeutic drug influences bladder activity in rats. | The Journal of urology 20080201 |
| Poor reproducibility of template bleeding time in horses. | Journal of veterinary internal medicine 20080101 |
| Effects of etamsylate on equine platelets: in vitro and in vivo studies. | Veterinary journal (London, England : 1997) 20070901 |
| Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. | Phytomedicine : international journal of phytotherapy and phytopharmacology 20070801 |
| Electrochemical parameters of ethamsylate at multi-walled carbon nanotube modified glassy carbon electrodes. | Bioelectrochemistry (Amsterdam, Netherlands) 20070501 |
| Minimising neonatal brain injury: how research in the past five years has changed my clinical practice. | Archives of disease in childhood 20070301 |
| Kinetic spectrophotometric determination of ethamsylate in dosage forms. | Journal of AOAC International 20070101 |
| [Hemostatic therapy for hemorrhages during first and second trimesters]. | Anesteziologiia i reanimatologiia 20070101 |
| Simultaneous determination of ethamsylate, tramadol and lidocaine in human urine by capillary electrophoresis with electrochemiluminescence detection. | Electrophoresis 20060901 |
| Therapeutic efficacy and mechanism of action of ethamsylate, a long-standing hemostatic agent. | American journal of therapeutics 20060101 |
| Single-blind, randomized controlled study of the clinical and urodynamic effects of an alpha-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. | The Journal of urology 20050901 |
| [Clinical study of Eviprostat for the treatment of benign prostatic hyperplasia]. | Zhonghua nan ke xue = National journal of andrology 20050901 |
| Etamsylate for prevention of periventricular haemorrhage. | Archives of disease in childhood. Fetal and neonatal edition 20050101 |
| Developmental outcome of the use of etamsylate for prevention of periventricular haemorrhage in a randomised controlled trial. | Archives of disease in childhood. Fetal and neonatal edition 20050101 |
| Sensitive kinetic spectrophotometric determination of captopril and ethamsylate in pharmaceutical preparations and biological fluids. | Farmaco (Societa chimica italiana : 1989) 20041001 |
| The hemostatic agent ethamsylate promotes platelet/leukocyte aggregate formation in a model of vascular injury. | Fundamental & clinical pharmacology 20040801 |
| Single-blind, randomized controlled study of the clinical and urodynamic effects of an alpha-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. | International journal of urology : official journal of the Japanese Urological Association 20040701 |
| Benefits and risks of pharmacological agents used for the treatment of menorrhagia. | Drug safety 20040101 |
| [The enzyme therapy within a complex treatment of hemophthalmos in patients with diabetes mellitus]. | Vestnik oftalmologii 20040101 |
| Vascular permeabilization by intravenous arachidonate in the rat peritoneal cavity: antagonism by ethamsylate. | European journal of pharmacology 20030411 |
| [BPH pharmacotherapy (miscellaneous)]. | Nihon rinsho. Japanese journal of clinical medicine 20021201 |
| Determination of ethamsylate in pharmaceutical preparations based on an auto-oxidation chemiluminescence reaction. | Journal of pharmaceutical and biomedical analysis 20021015 |
| The hemostatic agent ethamsylate enhances P-selectin membrane expression in human platelets and cultured endothelial cells. | Thrombosis research 20020915 |
| Bleeding from endometrial and vaginal malignant tumors treated with activated recombinant factor VII. | European journal of gynaecological oncology 20020101 |
| [State of the hemostasis system in Poland's syndrome]. | Vestnik khirurgii imeni I. I. Grekova 20020101 |
| Randomised controlled trial of prophylactic etamsylate: follow up at 2 years of age. | Archives of disease in childhood. Fetal and neonatal edition 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.