200,000+ products from a single source!
sales@angenechem.com
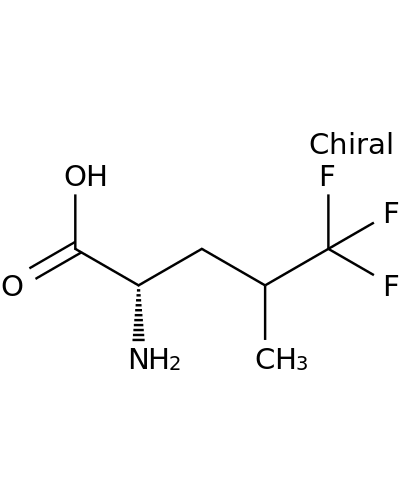
25367-71-9 | 5,5,5-Trifluoro-L-leucine
CAS No: 25367-71-9 Catalog No: AG0038UR MDL No:
Product Description
Catalog Number:
AG0038UR
Chemical Name:
5,5,5-Trifluoro-L-leucine
CAS Number:
25367-71-9
Molecular Formula:
C6H10F3NO2
Molecular Weight:
185.1443
IUPAC Name:
(2S)-2-amino-5,5,5-trifluoro-4-methylpentanoic acid
InChI:
InChI=1S/C6H10F3NO2/c1-3(6(7,8)9)2-4(10)5(11)12/h3-4H,2,10H2,1H3,(H,11,12)/t3?,4-/m0/s1
InChI Key:
XFGVJLGVINCWDP-BKLSDQPFSA-N
SMILES:
OC(=O)[C@H](CC(C(F)(F)F)C)N
Properties
Complexity:
169
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
185.066g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
185.146g/mol
Monoisotopic Mass:
185.066g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
63.3A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.9
Literature
| Title | Journal |
|---|---|
| Oxazoline-oxazinone oxidative rearrangement. divergent syntheses of (2S,3S)-4,4,4-trifluorovaline and (2S,4S)-5,5,5-Trifluoroleucine. | The Journal of organic chemistry 20090807 |
| Biosynthesis and stability of coiled-coil peptides containing (2S,4R)-5,5,5-trifluoroleucine and (2S,4S)-5,5,5-trifluoroleucine. | Chembiochem : a European journal of chemical biology 20090105 |
| Synthetic biology of proteins: tuning GFPs folding and stability with fluoroproline. | PloS one 20080101 |
| Influence of global fluorination on chloramphenicol acetyltransferase activity and stability. | Biotechnology and bioengineering 20060805 |
| Properties of a trifluoroleucine-resistant mutant of Saccharomyces cerevisiae. | Bioscience, biotechnology, and biochemistry 20060701 |
| Asp578 in LEU4p is one of the key residues for leucine feedback inhibition release in sake yeast. | Bioscience, biotechnology, and biochemistry 20050701 |
| Construction of two new vectors for transformation of laboratory, natural and industrial Saccharomyces cerevisiae strains to trifluoroleucine and G418 resistance. | Folia microbiologica 20040101 |
| The first isolation of two types of trifluoroleucine resistant mutants of Saccharomyces servazzii. | Biotechnology letters 20031001 |
| A simple and efficient method for the resolution of all four diastereomers of 4,4,4-trifluorovaline and 5,5,5-trifluoroleucine. | The Journal of organic chemistry 20020308 |
| Self-association and membrane-binding behavior of melittins containing trifluoroleucine. | Journal of the American Chemical Society 20010801 |
| A coiled coil with a fluorous core. | Journal of the American Chemical Society 20010516 |
| Stabilization of coiled-coil peptide domains by introduction of trifluoroleucine. | Biochemistry 20010306 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.