200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 2521-07-5
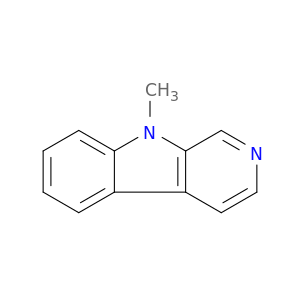
2521-07-5 | 9H-Pyrido[3,4-b]indole, 9-methyl-
CAS No: 2521-07-5 Catalog No: AG002QY4 MDL No:MFCD18803860
Product Description
Catalog Number:
AG002QY4
Chemical Name:
9H-Pyrido[3,4-b]indole, 9-methyl-
CAS Number:
2521-07-5
Molecular Formula:
C12H10N2
Molecular Weight:
182.2212
MDL Number:
MFCD18803860
IUPAC Name:
9-methylpyrido[3,4-b]indole
InChI:
InChI=1S/C12H10N2/c1-14-11-5-3-2-4-9(11)10-6-7-13-8-12(10)14/h2-8H,1H3
InChI Key:
MABOIYXDALNSES-UHFFFAOYSA-N
SMILES:
Cn1c2ccccc2c2c1cncc2
Properties
Complexity:
216
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
182.084g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
182.226g/mol
Monoisotopic Mass:
182.084g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.3
Literature
| Title | Journal |
|---|---|
| Good guys from a shady family. | Journal of neurochemistry 20120601 |
| 9-Methyl-β-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendritic and synaptic proliferation. | Journal of neurochemistry 20120601 |
| Photosensitization of DNA by β-carbolines: kinetic analysis and photoproduct characterization. | Organic & biomolecular chemistry 20120307 |
| Inhibition of the bioactivation of the neurotoxin MPTP by antioxidants, redox agents and monoamine oxidase inhibitors. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20110801 |
| Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: a new anti-Parkinson drug? | Expert review of neurotherapeutics 20110601 |
| The exceptional properties of 9-methyl-beta-carboline: stimulation, protection and regeneration of dopaminergic neurons coupled with anti-inflammatory effects. | Journal of neurochemistry 20100601 |
| Singlet excited state pyridinic deprotonation of the N9-methylbetacarboline cations in aqueous sodium hydroxide solutions. | Journal of fluorescence 20100101 |
| 9-Methyl-beta-carboline has restorative effects in an animal model of Parkinson's disease. | Pharmacological reports : PR 20100101 |
| A theoretical study of the hydrogen bond donor capability and co-operative effects in the hydrogen bond complexes of the diaza-aromatic betacarbolines. | Physical chemistry chemical physics : PCCP 20100101 |
| Ground and singlet excited state pyridinic protonation of N9-methylbetacarboline in water-N,N-dimethylformamide mixtures. | Journal of fluorescence 20091101 |
| Dual emission of temperature-induced betacarboline self-associated hydrogen bond aggregates. | Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 20090301 |
| 9-Methyl-beta-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. | Neurochemistry international 20080101 |
| Phenylethanolamine N-methyltransferase has beta-carboline 2N-methyltransferase activity: hypothetical relevance to Parkinson's disease. | Neurochemistry international 20020601 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.