200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2500-59-6
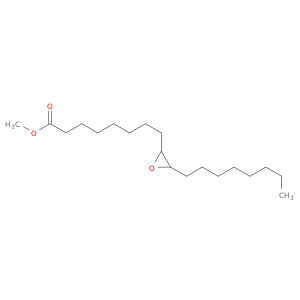
2500-59-6 | 2-Oxiraneoctanoic acid, 3-octyl-, methyl ester
CAS No: 2500-59-6 Catalog No: AG002Q68 MDL No:
Product Description
Catalog Number:
AG002Q68
Chemical Name:
2-Oxiraneoctanoic acid, 3-octyl-, methyl ester
CAS Number:
2500-59-6
Molecular Formula:
C19H36O3
Molecular Weight:
312.4873
IUPAC Name:
methyl 8-(3-octyloxiran-2-yl)octanoate
InChI:
InChI=1S/C19H36O3/c1-3-4-5-6-8-11-14-17-18(22-17)15-12-9-7-10-13-16-19(20)21-2/h17-18H,3-16H2,1-2H3
InChI Key:
CAMHHLOGFDZBBG-UHFFFAOYSA-N
SMILES:
CCCCCCCCC1OC1CCCCCCCC(=O)OC
EC Number:
219-699-0
Properties
Complexity:
278
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
312.266g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
312.494g/mol
Monoisotopic Mass:
312.266g/mol
Rotatable Bond Count:
16
Topological Polar Surface Area:
38.8A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
6.5
Literature
| Title | Journal |
|---|---|
| Evaporative light scattering detector in normal-phase high-performance liquid chromatography determination of FAME oxidation products. | Journal of chromatography. A 20120907 |
| An octanuclear molybdenum(VI) complex containing coordinatively bound 4,4'-di-tert-butyl-2,2'-bipyridine, [Mo8O22(OH)4(di-tBu-bipy)4]: synthesis, structure, and catalytic epoxidation of bio-derived olefins. | Inorganic chemistry 20120319 |
| A peroxygenase pathway involved in the biosynthesis of epoxy fatty acids in oat. | Plant physiology 20110901 |
| Synthesis of an amine-oleate derivative using an ionic liquid catalyst. | Journal of agricultural and food chemistry 20090923 |
| Photochemistry of imidacloprid in model systems. | Journal of agricultural and food chemistry 20080910 |
| Analysis of the oxidation products of cis- and trans-octadecenoate methyl esters by capillary gas chromatography-ion-trap mass spectrometry. I. Epoxide and dimeric compounds. | Journal of chromatography. A 20030124 |
Related Products
© 2019 Angene International Limited. All rights Reserved.