200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 247-66-5
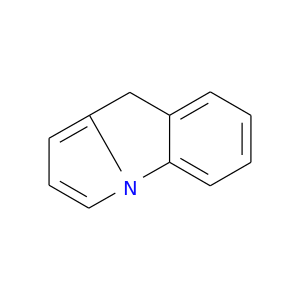
247-66-5 | 9H-Pyrrolo[1,2-a]indole
CAS No: 247-66-5 Catalog No: AG002P2N MDL No:
Product Description
Catalog Number:
AG002P2N
Chemical Name:
9H-Pyrrolo[1,2-a]indole
CAS Number:
247-66-5
Molecular Formula:
C11H9N
Molecular Weight:
155.1959
IUPAC Name:
4H-pyrrolo[1,2-a]indole
InChI:
InChI=1S/C11H9N/c1-2-6-11-9(4-1)8-10-5-3-7-12(10)11/h1-7H,8H2
InChI Key:
JGCYQWJGSSQVSZ-UHFFFAOYSA-N
SMILES:
c1ccc2c(c1)Cc1n2ccc1
Properties
Complexity:
178
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
155.073g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
155.2g/mol
Monoisotopic Mass:
155.073g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
4.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| Intramolecular charge transfer with fluorazene and N-phenylpyrrole. | The journal of physical chemistry. A 20100204 |
| Expedient synthesis of pyrrolo[1,2-a]indoles: preparation of the core of yuremamine. | Organic letters 20080821 |
| Chemistry of pyrrolo[1,2-a]indole- and pyrido[1,2-a]indole-based quinone methides. Mechanistic explanations for differences in cytostatic/cytotoxic properties. | The Journal of organic chemistry 20071109 |
| Evaluation of the anti-inflammatory and anti-nociceptive activities of novel synthesized melatonin analogues. | European journal of medicinal chemistry 20071001 |
| Design of a cyclopropyl quinone methide reductive alkylating agent. 2. | The Journal of organic chemistry 20060804 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







