200,000+ products from a single source!
sales@angenechem.com
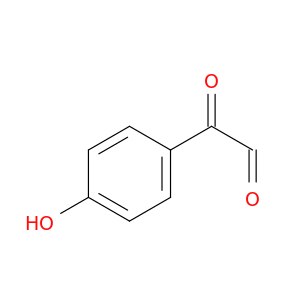
24645-80-5 | Benzeneacetaldehyde, 4-hydroxy-α-oxo-
CAS No: 24645-80-5 Catalog No: AG002OX8 MDL No:MFCD00054984
Product Description
Catalog Number:
AG002OX8
Chemical Name:
Benzeneacetaldehyde, 4-hydroxy-α-oxo-
CAS Number:
24645-80-5
Molecular Formula:
C8H6O3
Molecular Weight:
150.1314
MDL Number:
MFCD00054984
IUPAC Name:
2-(4-hydroxyphenyl)-2-oxoacetaldehyde
InChI:
InChI=1S/C8H6O3/c9-5-8(11)6-1-3-7(10)4-2-6/h1-5,10H
InChI Key:
MTMONFVFAYLRSG-UHFFFAOYSA-N
SMILES:
O=CC(=O)c1ccc(cc1)O
UNII:
APL7XFW2OP
NSC Number:
145743
Properties
Complexity:
157
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
150.032g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
150.133g/mol
Monoisotopic Mass:
150.032g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
54.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.4
Literature
| Title | Journal |
|---|---|
| Revealing a positive charge patch on a recombinant monoclonal antibody by chemical labeling and mass spectrometry. | Analytical chemistry 20111115 |
| Cross-talk between the catalytic core and the regulatory domain in cystathionine β-synthase: study by differential covalent labeling and computational modeling. | Biochemistry 20101214 |
| A technique for the specific enrichment of citrulline-containing peptides. | Analytical biochemistry 20100801 |
| Probing the ligand-induced conformational change in HLA-DR1 by selective chemical modification and mass spectrometric mapping. | Biochemistry 20051025 |
| Alteration of substrate selectivity through mutation of two arginine residues in the binding site of amadoriase II from Aspergillus sp. | Biochemistry 20020402 |
| Ligand-selective modulation of the permeability transition pore by arginine modification. Opposing effects of p-hydroxyphenylglyoxal and phenylglyoxal. | The Journal of biological chemistry 20020111 |
Related Products
© 2019 Angene International Limited. All rights Reserved.