200,000+ products from a single source!
sales@angenechem.com
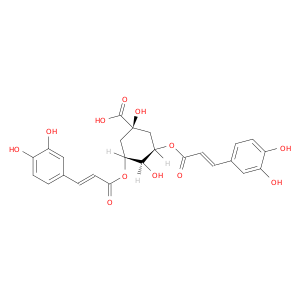
2450-53-5 | Isochlorogenic acid A
CAS No: 2450-53-5 Catalog No: AG0039M8 MDL No:MFCD00951290
Product Description
Catalog Number:
AG0039M8
Chemical Name:
Isochlorogenic acid A
CAS Number:
2450-53-5
Molecular Formula:
C25H24O12
Molecular Weight:
516.4509
MDL Number:
MFCD00951290
IUPAC Name:
(3R,5R)-3,5-bis[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy]-1,4-dihydroxycyclohexane-1-carboxylic acid
InChI:
InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(30)36-19-11-25(35,24(33)34)12-20(23(19)32)37-22(31)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-29,32,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23?,25?/m1/s1
InChI Key:
KRZBCHWVBQOTNZ-RDJMKVHDSA-N
SMILES:
O=C(O[C@@H]1C[C@@](O)(C[C@H]([C@H]1O)OC(=O)/C=C/c1ccc(c(c1)O)O)C(=O)O)/C=C/c1ccc(c(c1)O)O
UNII:
ND94C5E75K
Properties
Complexity:
825
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
2
Exact Mass:
516.127g/mol
Formal Charge:
0
Heavy Atom Count:
37
Hydrogen Bond Acceptor Count:
12
Hydrogen Bond Donor Count:
7
Isotope Atom Count:
0
Molecular Weight:
516.455g/mol
Monoisotopic Mass:
516.127g/mol
Rotatable Bond Count:
9
Topological Polar Surface Area:
211A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.5
Literature
| Title | Journal |
|---|---|
| Incorporation of Privileged Structures into Bevirimat Can Improve Activity against Wild-Type and Bevirimat-Resistant HIV-1. | Journal of medicinal chemistry 20161013 |
| 3,5-Dicaffeoylquinic acid isolated from Artemisia argyi and its ester derivatives exert anti-leucyl-tRNA synthetase of Giardia lamblia (GlLeuRS) and potential anti-giardial effects. | Fitoterapia 20121001 |
| Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. | Bioorganic & medicinal chemistry 20121001 |
| Metabolomic profiling of the flower bud and rachis of Tussilago farfara with antitussive and expectorant effects on mice. | Journal of ethnopharmacology 20120306 |
| Antioxidant activity-guided phytochemical investigation of Artemisia gmelinii Webb. ex Stechm.: isolation and spectroscopic challenges of 3,5-O-dicaffeoyl (epi?) quinic acid and its ethyl ester. | Journal of pharmaceutical and biomedical analysis 20120205 |
| Design, synthesis and evaluation of caffeic acid phenethyl ester-based inhibitors targeting a selectivity pocket in the active site of human aldo-keto reductase 1B10. | European journal of medicinal chemistry 20120201 |
| Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-κB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and -3 in human colon cancer cells. | Molecular nutrition & food research 20111001 |
| Youngia denticulata protects against oxidative damage induced by tert-butylhydroperoxide in HepG2 cells. | Journal of medicinal food 20111001 |
| Phenolic substances from Phagnalon rupestre protect against 2,4,6-trinitrochlorobenzene-induced contact hypersensitivity. | Journal of natural products 20110527 |
| Natural products from Scorzonera aristata (Asteraceae). | Natural product communications 20100501 |
| Synthesis, anti-HIV and anti-oxidant activities of caffeoyl 5,6-anhydroquinic acid derivatives. | Bioorganic & medicinal chemistry 20100115 |
| Preparative separation of isomeric caffeoylquinic acids from Flos Lonicerae by pH-zone-refining counter-current chromatography. | Journal of chromatography. A 20081128 |
| Synthesis and antiviral properties of some polyphenols related to Salvia genus. | Bioorganic & medicinal chemistry letters 20080815 |
| Simultaneous determination of 13 bioactive compounds in Herba Artemisiae Scopariae (Yin Chen) from different harvest seasons by HPLC-DAD. | Journal of pharmaceutical and biomedical analysis 20080805 |
| In vitro hepatoprotective compounds from Suaeda glauca. | Archives of pharmacal research 20080501 |
| Effects of plant alkylphenols on cytokine production, tyrosine nitration and inflammatory damage in the efferent phase of contact hypersensitivity. | British journal of pharmacology 20071001 |
| Targeted natural product isolation guided by HPLC-SPE-NMR: constituents of Hubertia species. | Journal of natural products 20070901 |
| Prevention of lipopolysaccharide-induced injury by 3,5-dicaffeoylquinic acid in endothelial cells. | Acta pharmacologica Sinica 20070801 |
| Preparative isolation and purification of dicaffeoylquinic acids from the Ainsliaea fragrans champ by high-speed counter-current chromatography. | Phytochemical analysis : PCA 20070101 |
| Determination of active constituents in Lonicera confusa DC. by capillary electrophoresis with amperometric detection. | Biomedical chromatography : BMC 20061101 |
| Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). | Journal of ethnopharmacology 20060630 |
| Vanillic acid glycoside and quinic acid derivatives from Gardeniae Fructus. | Journal of natural products 20060401 |
| A novel inhibitor of respiratory syncytial virus isolated from ethnobotanicals. | Antiviral research 20051201 |
| Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. | Phytotherapy research : PTR 20050301 |
| Anti-HIV activities of natural antioxidant caffeic acid derivatives: toward an antiviral supplementation diet. | Current medicinal chemistry 20050101 |
| (-)-3,5-Dicaffeoyl-muco-quinic acid isolated from Aster scaber contributes to the differentiation of PC12 cells: through tyrosine kinase cascade signaling. | Biochemical and biophysical research communications 20040123 |
| Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius). | Journal of agricultural and food chemistry 20030129 |
| Identification of isomeric dicaffeoylquinic acids from Eleutherococcus senticosus using HPLC-ESI/TOF/MS and 1H-NMR methods. | Phytochemical analysis : PCA 20020101 |
| A new caffeoyl quinic acid from aster scaber and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. | Chemical & pharmaceutical bulletin 20001101 |
| Structure-activity relationships: analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. | Journal of medicinal chemistry 19990211 |
| Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase. | Antimicrobial agents and chemotherapy 19980101 |
| Dicaffeoylquinic acid inhibitors of human immunodeficiency virus integrase: inhibition of the core catalytic domain of human immunodeficiency virus integrase. | Molecular pharmacology 19961001 |
| Inhibitors of HIV-1 replication [corrected; erratum to be published] that inhibit HIV integrase. | Proceedings of the National Academy of Sciences of the United States of America 19960625 |
| Differential inhibition of reverse transcriptase and cellular DNA polymerase-alpha activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. | Antiviral research 19950801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.