200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 241-55-4
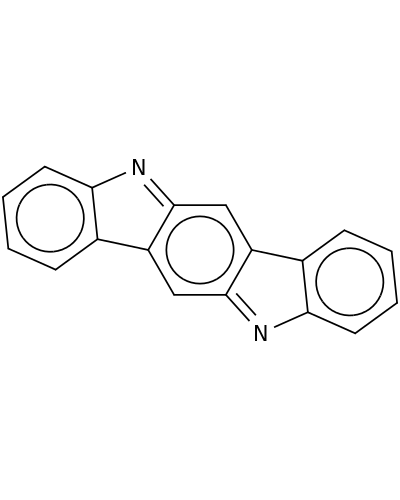
241-55-4 | indolo(3,2-b)carbazole
CAS No: 241-55-4 Catalog No: AG006X6X MDL No:
Product Description
Catalog Number:
AG006X6X
Chemical Name:
indolo(3,2-b)carbazole
CAS Number:
241-55-4
Molecular Formula:
C18H10N2
Molecular Weight:
254.2854
IUPAC Name:
indolo[3,2-b]carbazole
InChI:
InChI=1S/C18H10N2/c1-3-7-15-11(5-1)13-9-18-14(10-17(13)19-15)12-6-2-4-8-16(12)20-18/h1-10H
InChI Key:
HLVSZSQYBQCBQG-UHFFFAOYSA-N
SMILES:
c1ccc2c(c1)c1cc3=Nc4c(c3cc1=N2)cccc4
Properties
Complexity:
526
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
254.084g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
254.292g/mol
Monoisotopic Mass:
254.084g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
24.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| Hepatic Aryl Hydrocarbon Receptor Attenuates Fibroblast Growth Factor 21 Expression. | The Journal of biological chemistry 20160715 |
| Aryl Hydrocarbon Receptor Activation Synergistically Induces Lipopolysaccharide-Mediated Expression of Proinflammatory Chemokine (c-c motif) Ligand 20. | Toxicological sciences : an official journal of the Society of Toxicology 20151101 |
| Comparisons of differential gene expression elicited by TCDD, PCB126, βNF, or ICZ in mouse hepatoma Hepa1c1c7 cells and C57BL/6 mouse liver. | Toxicology letters 20131023 |
| Aryl hydrocarbon receptor-dependent cell cycle arrest in isolated mouse oval cells. | Toxicology letters 20131023 |
| Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit Toll-like receptor-induced maturation in monocyte-derived dendritic cells. | The British journal of dermatology 20120901 |
| Indolocarbazole-based ligands for ladder-type four-coordinate boron complexes. | Organic letters 20120706 |
| High-performance organic single-crystal field-effect transistors of indolo[3,2-b]carbazole and their potential applications in gas controlled organic memory devices. | Advanced materials (Deerfield Beach, Fla.) 20111116 |
| Charge transport in organic crystals: role of disorder and topological connectivity. | Journal of the American Chemical Society 20100825 |
| Diverse chemicals including aryl hydrocarbon receptor ligands modulate transcriptional activity of the 3'immunoglobulin heavy chain regulatory region. | Toxicology 20090630 |
| A human intervention study with foods containing natural Ah-receptor agonists does not significantly show AhR-mediated effects as measured in blood cells and urine. | Chemico-biological interactions 20081022 |
| Facile synthesis of novel indolo[3,2-b]carbazole derivatives and a chromogenic-sensing 5,12-dihydroindolo[3,2-b]carbazole. | Organic & biomolecular chemistry 20080721 |
| AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. | The Journal of investigative dermatology 20080701 |
| Gene expression profiling in Caco-2 human colon cells exposed to TCDD, benzo[a]pyrene, and natural Ah receptor agonists from cruciferous vegetables and citrus fruits. | Toxicology in vitro : an international journal published in association with BIBRA 20080301 |
| Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. | Toxicological sciences : an official journal of the Society of Toxicology 20070401 |
| Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. | Carcinogenesis 20051001 |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces suppressor of cytokine signaling 2 in murine B cells. | Molecular pharmacology 20041201 |
| Biological targets of antitumor indolocarbazoles bearing a sugar moiety. | Current medicinal chemistry. Anti-cancer agents 20041101 |
| Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. | Chemico-biological interactions 20041015 |
| Positive and negative regulation of prostaglandin E2 biosynthesis in human colorectal carcinoma cells by cancer chemopreventive agents. | Biochemical pharmacology 20030701 |
| Differential effects of vegetable-derived indoles on the induction of quinone reductase in hepatoma cells. | Journal of nutritional science and vitaminology 20021201 |
| Indolo[3,2-b]carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumor promoter. | Carcinogenesis 20021101 |
| Comparison of acute toxicities of indolo[3,2-b]carbazole (ICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in TCDD-sensitive rats. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20020701 |
| The biosynthetic gene cluster for the antitumor rebeccamycin: characterization and generation of indolocarbazole derivatives. | Chemistry & biology 20020401 |
| Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. | The Journal of general virology 20010601 |
| Synthesis and biological activities of indolocarbazoles bearing amino acid residues. | European journal of medicinal chemistry 20010101 |
| Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. | Proceedings of the National Academy of Sciences of the United States of America 19960319 |
| Regulation of CYP1A1 by indolo[3,2-b]carbazole in murine hepatoma cells. | The Journal of biological chemistry 19950922 |
| Regulation of human dioxin receptor function by indolocarbazoles, receptor ligands of dietary origin. | The Journal of biological chemistry 19940218 |
| Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. | Proceedings of the National Academy of Sciences of the United States of America 19911101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







