200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 2380-84-9
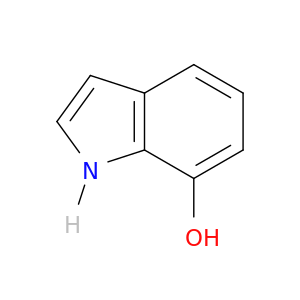
2380-84-9 | 1H-Indol-7-ol
CAS No: 2380-84-9 Catalog No: AG006UHT MDL No:MFCD00152102
Product Description
Catalog Number:
AG006UHT
Chemical Name:
1H-Indol-7-ol
CAS Number:
2380-84-9
Molecular Formula:
C8H7NO
Molecular Weight:
133.1473
MDL Number:
MFCD00152102
IUPAC Name:
1H-indol-7-ol
InChI:
InChI=1S/C8H7NO/c10-7-3-1-2-6-4-5-9-8(6)7/h1-5,9-10H
InChI Key:
ORVPXPKEZLTMNW-UHFFFAOYSA-N
SMILES:
Oc1cccc2c1[nH]cc2
UNII:
OR3V96LY5G
Properties
Complexity:
126
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
133.053g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
133.15g/mol
Monoisotopic Mass:
133.053g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
36A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| A microfluidic device for high throughput bacterial biofilm studies. | Lab on a chip 20120321 |
| The Pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. | PloS one 20120101 |
| Collagen-like proteins in pathogenic E. coli strains. | PloS one 20120101 |
| Characterization of a novel phenol hydroxylase in indoles biotransformation from a strain Arthrobacter sp. W1 [corrected]. | PloS one 20120101 |
| Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. | BMC microbiology 20110101 |
| Inhibitory effect of hydroxyindoles and their analogues on human melanoma tyrosinase. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20100101 |
| Patterning of mutually interacting bacterial bodies: close contacts and airborne signals. | BMC microbiology 20100101 |
| Synthesis of the marine pyrroloiminoquinone alkaloids, discorhabdins. | Marine drugs 20100101 |
| Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. | Microbial biotechnology 20090101 |
| Multicomponent phenol hydroxylase-catalysed formation of hydroxyindoles and dyestuffs from indole and its derivatives. | Letters in applied microbiology 20050101 |
| Microbial hydroxylation of indole to 7-hydroxyindole by Acinetobacter calcoaceticus strain 4-1-5. | Bioscience, biotechnology, and biochemistry 20040501 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.