200,000+ products from a single source!
sales@angenechem.com
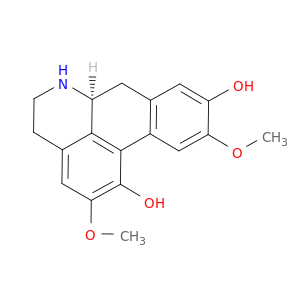
23599-69-1 | 4H-Dibenzo[de,g]quinoline-1,9-diol, 5,6,6a,7-tetrahydro-2,10-dimethoxy-, (6aS)-
CAS No: 23599-69-1 Catalog No: AG002NU2 MDL No:MFCD09953815
Product Description
Catalog Number:
AG002NU2
Chemical Name:
4H-Dibenzo[de,g]quinoline-1,9-diol, 5,6,6a,7-tetrahydro-2,10-dimethoxy-, (6aS)-
CAS Number:
23599-69-1
Molecular Formula:
C18H19NO4
Molecular Weight:
313.3478
MDL Number:
MFCD09953815
IUPAC Name:
(6aS)-2,10-dimethoxy-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-1,9-diol
InChI:
InChI=1S/C18H19NO4/c1-22-14-8-11-10(6-13(14)20)5-12-16-9(3-4-19-12)7-15(23-2)18(21)17(11)16/h6-8,12,19-21H,3-5H2,1-2H3/t12-/m0/s1
InChI Key:
HORZNQYQXBFWNZ-LBPRGKRZSA-N
SMILES:
COc1cc2c(cc1O)C[C@H]1c3c2c(O)c(cc3CCN1)OC
Properties
Complexity:
433
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
313.131g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
313.353g/mol
Monoisotopic Mass:
313.131g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
71A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.3
Literature
| Title | Journal |
|---|---|
| Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. | The international journal of biochemistry & cell biology 20160601 |
| Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. | Toxicology and applied pharmacology 20150101 |
| Norisoboldine, an Anti-Arthritis Alkaloid Isolated from Radix Linderae, Attenuates Osteoclast Differentiation and Inflammatory Bone Erosion in an Aryl Hydrocarbon Receptor-Dependent Manner. | International journal of biological sciences 20150101 |
| Norisoboldine inhibits the production of interleukin-6 in fibroblast-like synoviocytes from adjuvant arthritis rats through PKC/MAPK/NF-κB-p65/CREB pathways. | Journal of cellular biochemistry 20120801 |
| Norisoboldine, an alkaloid compound isolated from Radix Linderae, inhibits synovial angiogenesis in adjuvant-induced arthritis rats by moderating Notch1 pathway-related endothelial tip cell phenotype. | Experimental biology and medicine (Maywood, N.J.) 20120801 |
| Simultaneous determination of synephrine, arecoline, and norisoboldine in Chinese patent medicine Si-Mo-Tang oral liquid preparation by strong cation exchange high performance liquid chromatography. | Pharmaceutical biology 20120701 |
| Simultaneous determination of norisoboldine and its major metabolite in rat plasma by ultra-performance liquid chromatography-mass spectrometry and its application in a pharmacokinetic study. | Biomedical chromatography : BMC 20110301 |
| Norisoboldine inhibits the production of pro-inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 cells by down-regulating the activation of MAPKs but not NF-κB. | Inflammation 20101201 |
| Characterization of new metabolites from in vivo biotransformation of norisoboldine by liquid chromatography/mass spectrometry and NMR spectroscopy. | Journal of pharmaceutical and biomedical analysis 20100905 |
| Therapeutic effect of norisoboldine, an alkaloid isolated from Radix Linderae, on collagen-induced arthritis in mice. | Phytomedicine : international journal of phytotherapy and phytopharmacology 20100801 |
| [Determination of norisoboldine in Radix Lindera by RP-HPLC]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20091101 |
| [Evaluation of antioxidant activity of Radix Linderae and other two Chinese drugs using TLC-bioautography]. | Yao xue xue bao = Acta pharmaceutica Sinica 20061001 |
| Geographic distribution of three alkaloid chemotypes of Croton lechleri. | Journal of natural products 20020601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.