200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2348-82-5
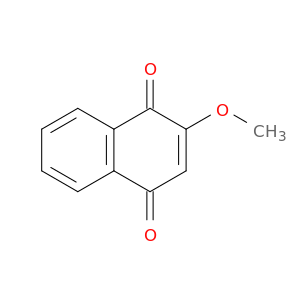
2348-82-5 | 1,4-Naphthalenedione, 2-methoxy-
CAS No: 2348-82-5 Catalog No: AG002NDL MDL No:MFCD00019539
Product Description
Catalog Number:
AG002NDL
Chemical Name:
1,4-Naphthalenedione, 2-methoxy-
CAS Number:
2348-82-5
Molecular Formula:
C11H8O3
Molecular Weight:
188.1794
MDL Number:
MFCD00019539
IUPAC Name:
2-methoxynaphthalene-1,4-dione
InChI:
InChI=1S/C11H8O3/c1-14-10-6-9(12)7-4-2-3-5-8(7)11(10)13/h2-6H,1H3
InChI Key:
OBGBGHKYJAOXRR-UHFFFAOYSA-N
SMILES:
COC1=CC(=O)c2c(C1=O)cccc2
UNII:
39020BUT1D
NSC Number:
31530
Properties
Complexity:
304
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
188.047g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
188.182g/mol
Monoisotopic Mass:
188.047g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
43.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.4
Literature
| Title | Journal |
|---|---|
| 2-Methoxy-1,4-Naphthoquinone (MNQ) suppresses the invasion and migration of a human metastatic breast cancer cell line (MDA-MB-231). | Toxicology in vitro : an international journal published in association with BIBRA 20140401 |
| New oxirane derivatives of 1,4-naphthoquinones and their evaluation against T. cruzi epimastigote forms. | Bioorganic & medicinal chemistry 20120815 |
| 2-Meth-oxy-naphthalene-1,4-dione. | Acta crystallographica. Section E, Structure reports online 20110401 |
| In Vitro Activity of 2-methoxy-1,4-naphthoquinone and Stigmasta-7,22-diene-3β-ol from Impatiens balsamina L. against Multiple Antibiotic-Resistant Helicobacter pylori. | Evidence-based complementary and alternative medicine : eCAM 20110101 |
| Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. | Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 20110101 |
| 2-methoxy-1,4-naphthoquinone isolated from Impatiens balsamina in a screening program for activity to inhibit Wnt signaling. | Journal of natural medicines 20110101 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Substituted naphthoquinones as novel amino acid sensitive reagents for the detection of latent fingermarks on paper surfaces. | Talanta 20101015 |
| Naphthoquinones and anthraquinones from scent glands of a dyspnoid Harvestman, Paranemastoma quadripunctatum. | Journal of chemical ecology 20100201 |
| Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. | Journal of medicinal chemistry 20080327 |
| Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. | Molecules (Basel, Switzerland) 20080131 |
| Effects of the compounds 2-methoxynaphthoquinone, 2-propoxynaphthoquinone, and 2-isopropoxynaphthoquinone on ecdysone 20-monooxygenase activity. | Archives of insect biochemistry and physiology 20070901 |
| Structure-activity relationships in the haemolytic activity and nephrotoxicity of derivatives of 1,2- and 1,4-naphthoquinone. | Journal of applied toxicology : JAT 20070101 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Design, synthesis, and biological evaluation of prazosin-related derivatives as multipotent compounds. | Journal of medicinal chemistry 20050113 |
| Photoreactions of 1,4-Naphthoquinones: effects of substituents and water on the intermediates and reactivity. | Photochemistry and photobiology 20050101 |
| Role of oxidant stress in lawsone-induced hemolytic anemia. | Toxicological sciences : an official journal of the Society of Toxicology 20041201 |
| Discovery, total synthesis, HRV 3C-protease inhibitory activity, and structure-activity relationships of 2-methoxystypandrone and its analogues. | Bioorganic & medicinal chemistry letters 20011217 |
| Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation. | Phytotherapy research : PTR 20011201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







