200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 234-95-7
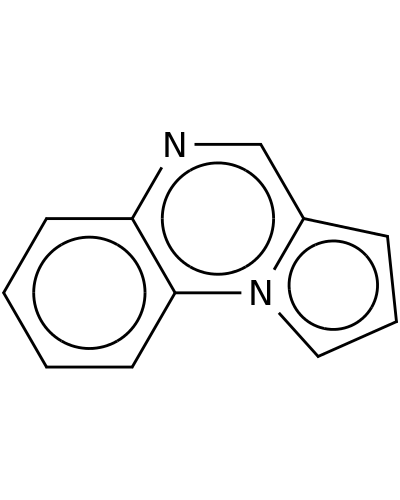
234-95-7 | Pyrrolo[1,2-a]quinoxaline
CAS No: 234-95-7 Catalog No: AG002N3V MDL No:MFCD00004950
Product Description
Catalog Number:
AG002N3V
Chemical Name:
Pyrrolo[1,2-a]quinoxaline
CAS Number:
234-95-7
Molecular Formula:
C11H8N2
Molecular Weight:
168.1946
MDL Number:
MFCD00004950
IUPAC Name:
pyrrolo[1,2-a]quinoxaline
InChI:
InChI=1S/C11H8N2/c1-2-6-11-10(5-1)12-8-9-4-3-7-13(9)11/h1-8H
InChI Key:
IEOUSWADWJLLCH-UHFFFAOYSA-N
SMILES:
c1ccc2c(c1)n1cccc1cn2
EC Number:
205-942-8
NSC Number:
106795
Properties
Complexity:
193
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
168.069g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
168.199g/mol
Monoisotopic Mass:
168.069g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.9
Literature
| Title | Journal |
|---|---|
| Pyrrolo[1,2-a]quinoxalines: novel synthesis via annulation of 2-alkylquinoxalines. | Organic letters 20121005 |
| One-pot synthesis of pyrrolo[1,2-a]quinoxaline derivatives via iron-promoted aryl nitro reduction and aerobic oxidation of alcohols. | Organic letters 20120921 |
| One-pot synthesis of pyrrolo[1,2-a]quinoxalines. | Organic & biomolecular chemistry 20111107 |
| New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity--Part II. | European journal of medicinal chemistry 20110601 |
| Synthesis of Pyrrolo[1,2-a]quinoxalines via gold(I)-mediated cascade reactions. | ACS combinatorial science 20110509 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Pt(IV)-catalyzed hydroamination triggered cyclization: a strategy to fused pyrrolo[1,2-a]quinoxalines, indolo[1,2-a]quinoxalines, and indolo[3,2-c]quinolines. | The Journal of organic chemistry 20100521 |
| Synthesis and evaluation of the antiproliferative activity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt kinase. Part II. | Journal of enzyme inhibition and medicinal chemistry 20100401 |
| New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity. | Bioorganic & medicinal chemistry 20081015 |
| Synthesis of new pyrrolo[1,2-a]quinoxaline derivatives as potential inhibitors of Akt kinase. | Journal of enzyme inhibition and medicinal chemistry 20081001 |
| A one-pot coupling/hydrolysis/condensation process to pyrrolo[1,2-a]quinoxaline. | The Journal of organic chemistry 20080704 |
| Synthesis of new 4-(E)-alkenylpyrrolo[1,2-a]quinoxalines as antileishmanial agents by Suzuki-Miyaura cross-coupling reactions. | Journal of enzyme inhibition and medicinal chemistry 20071001 |
| Synthesis of new 4-[2-(alkylamino) ethylthio]pyrrolo[1,2-a]quinoxaline and 5-[2-(alkylamino) ethylthio]pyrrolo[1,2-a]thieno[3,2-e]pyrazine derivatives, as potential bacterial multidrug resistance pump inhibitors. | Journal of enzyme inhibition and medicinal chemistry 20071001 |
| Synthesis, analytical behaviour and biological evaluation of new 4-substituted pyrrolo[1,2-a]quinoxalines as antileishmanial agents. | Bioorganic & medicinal chemistry 20070101 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.