200,000+ products from a single source!
sales@angenechem.com
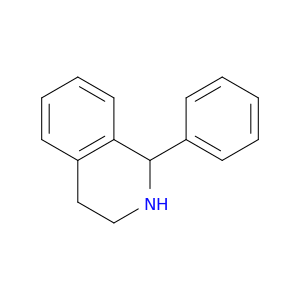
22990-19-8 | Isoquinoline, 1,2,3,4-tetrahydro-1-phenyl-
CAS No: 22990-19-8 Catalog No: AG002LS1 MDL No:MFCD02179241
Product Description
Catalog Number:
AG002LS1
Chemical Name:
Isoquinoline, 1,2,3,4-tetrahydro-1-phenyl-
CAS Number:
22990-19-8
Molecular Formula:
C15H15N
Molecular Weight:
209.2863
MDL Number:
MFCD02179241
IUPAC Name:
1-phenyl-1,2,3,4-tetrahydroisoquinoline
InChI:
InChI=1S/C15H15N/c1-2-7-13(8-3-1)15-14-9-5-4-6-12(14)10-11-16-15/h1-9,15-16H,10-11H2
InChI Key:
PRTRSEDVLBBFJZ-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)C1NCCc2c1cccc2
NSC Number:
338399
Properties
Complexity:
220
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
209.12g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
209.292g/mol
Monoisotopic Mass:
209.12g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
12A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Synthesis and biological evaluation of 1-phenyl-1,2,3,4-dihydroisoquinoline compounds as tubulin polymerization inhibitors. | Archiv der Pharmazie 20120601 |
| Ortho-rhom-bic polymorph of (6,7-dimeth-oxy-1,2,3,4-tetra-hydro-isoquinolin-1-yl)methanol. | Acta crystallographica. Section E, Structure reports online 20110701 |
| Synthesis and in vitro cytotoxicity of 1,2,3,4-tetrahydroisoquinoline derivatives. | European journal of medicinal chemistry 20060201 |
| In situ selection of lead compounds by click chemistry: target-guided optimization of acetylcholinesterase inhibitors. | Journal of the American Chemical Society 20050511 |
| Design, synthesis, and inhibition of platelet aggregation for some 1-o-chlorophenyl-1,2,3,4-tetrahydroisoquinoline derivatives. | Bioorganic & medicinal chemistry 20041215 |
| Evaluation of the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats. | Psychopharmacology 20031101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.