200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2235-15-6
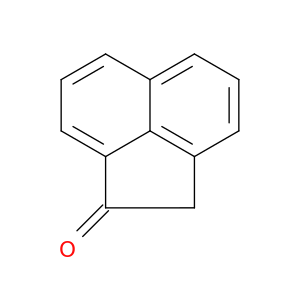
2235-15-6 | Acenaphthylen-1(2H)-one
CAS No: 2235-15-6 Catalog No: AG003DVY MDL No:MFCD00156651
Product Description
Catalog Number:
AG003DVY
Chemical Name:
Acenaphthylen-1(2H)-one
CAS Number:
2235-15-6
Molecular Formula:
C12H8O
Molecular Weight:
168.1913
MDL Number:
MFCD00156651
IUPAC Name:
2H-acenaphthylen-1-one
InChI:
InChI=1S/C12H8O/c13-11-7-9-5-1-3-8-4-2-6-10(11)12(8)9/h1-6H,7H2
InChI Key:
JBXIOAKUBCTDES-UHFFFAOYSA-N
SMILES:
O=C1Cc2c3c1cccc3ccc2
UNII:
81QD8CCK3V
Properties
Complexity:
233
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
168.058g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
168.195g/mol
Monoisotopic Mass:
168.058g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.6
Literature
| Title | Journal |
|---|---|
| Ethyl (1R,1'S,2'S,7a'R)-2-oxo-1'-[(3aR,5R,5aS,8aS,8bR)-2,2,7,7-tetra-methyl-tetra-hydro-3aH-bis-[1,3]dioxolo[4,5-b:4',5'-d]pyran-5-yl]-1',2',5',6',7',7a'-hexa-hydro-2H-spiro-[acenaphthyl-ene-1,3'-pyrrolizine]-2'-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20120201 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| 3'-Benzoyl-1'-methyl-4'-phenyl-spiro[acenaphthyl-ene-1(2H),2'-pyrrolidin]-2-one. | Acta crystallographica. Section E, Structure reports online 20101101 |
| Bacterial degradation of aromatic compounds. | International journal of environmental research and public health 20090101 |
| Ethyl 3'-cyano-1'-methyl-2-oxo-4'-phenylspiro-[acenaphthene-1,2'-pyrrolidine]-3'-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20080201 |
| Metabolism of fluoranthene by Mycobacterium sp. strain AP1. | Applied microbiology and biotechnology 20060501 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Degradation and formation of polycyclic aromatic compounds during bioslurry treatment of an aged gasworks soil. | Environmental toxicology and chemistry 20030701 |
| New methodologies for the preparation of porphodimethenes and their conversion to trans-porphyrins with functionalized naphthyl spacers. | The Journal of organic chemistry 20010810 |
| Convergent and divergent points in catabolic pathways involved in utilization of fluoranthene, naphthalene, anthracene, and phenanthrene by Sphingomonas paucimobilis var. EPA505. | Journal of industrial microbiology & biotechnology 20010601 |
| The electron as a protecting group. 3. Generation of acenaphthyne radical anion and the determination of the heat of formation of a strained cycloalkyne. | Journal of the American Chemical Society 20010509 |
| Use of 13C nuclear magnetic resonance to assess fossil fuel biodegradation: fate of [1-13C]acenaphthene in creosote polycyclic aromatic compound mixtures degraded by bacteria. | Applied and environmental microbiology 19980401 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.