200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2174-64-3
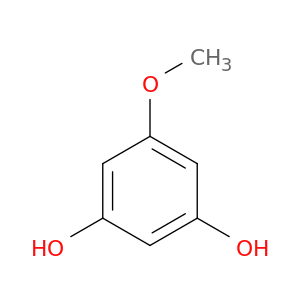
2174-64-3 | 3-Hydroxy-5-methoxyphenol
CAS No: 2174-64-3 Catalog No: AG0033JE MDL No:MFCD00002285
Product Description
Catalog Number:
AG0033JE
Chemical Name:
3-Hydroxy-5-methoxyphenol
CAS Number:
2174-64-3
Molecular Formula:
C7H8O3
Molecular Weight:
140.1366
MDL Number:
MFCD00002285
IUPAC Name:
5-methoxybenzene-1,3-diol
InChI:
InChI=1S/C7H8O3/c1-10-7-3-5(8)2-6(9)4-7/h2-4,8-9H,1H3
InChI Key:
HDVRLUFGYQYLFJ-UHFFFAOYSA-N
SMILES:
COc1cc(O)cc(c1)O
EC Number:
218-532-9
UNII:
6201E0JIF3
Properties
Complexity:
95
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
140.047g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
140.138g/mol
Monoisotopic Mass:
140.047g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
49.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.3
Literature
| Title | Journal |
|---|---|
| (2,4-Dihy-droxy-6-meth-oxy-phen-yl)(3,5-dihy-droxy-phen-yl)methanone monohydrate. | Acta crystallographica. Section E, Structure reports online 20111001 |
| 1-(2,6-Dihydr-oxy-4-methoxy-phen-yl)-3-phenyl-propan-1-one. | Acta crystallographica. Section E, Structure reports online 20100501 |
| Stopped-flow kinetic study of the aroxyl radical-scavenging action of catechins and vitamin C in ethanol and micellar solutions. | Journal of agricultural and food chemistry 20080625 |
| Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. | Free radical biology & medicine 20050915 |
| Structure-activity relationship of the tocopherol-regeneration reaction by catechins. | Free radical biology & medicine 20050501 |
| The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1,3,5-trimethoxybenzene. | Plant physiology 20040501 |
| Two O-methyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. | Journal of bioscience and bioengineering 20030101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.