200,000+ products from a single source!
sales@angenechem.com
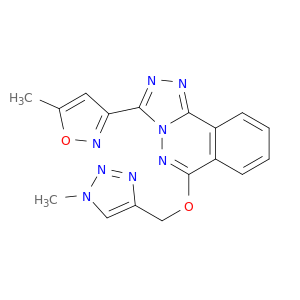
215874-86-5 | 3-(5-Methylisoxazol-3-yl)-6-[(1-methyl-1H-1,2,3-triazol-4-yl)methoxy][1,2,4]triazolo[3,4-a]phthalazine
CAS No: 215874-86-5 Catalog No: AG0033GL MDL No:MFCD09832717
Product Description
Catalog Number:
AG0033GL
Chemical Name:
3-(5-Methylisoxazol-3-yl)-6-[(1-methyl-1H-1,2,3-triazol-4-yl)methoxy][1,2,4]triazolo[3,4-a]phthalazine
CAS Number:
215874-86-5
Molecular Formula:
C17H14N8O2
Molecular Weight:
362.3455
MDL Number:
MFCD09832717
IUPAC Name:
5-methyl-3-[6-[(1-methyltriazol-4-yl)methoxy]-[1,2,4]triazolo[3,4-a]phthalazin-3-yl]-1,2-oxazole
InChI:
InChI=1S/C17H14N8O2/c1-10-7-14(22-27-10)16-20-19-15-12-5-3-4-6-13(12)17(21-25(15)16)26-9-11-8-24(2)23-18-11/h3-8H,9H2,1-2H3
InChI Key:
NZMJFRXKGUCYNP-UHFFFAOYSA-N
SMILES:
Cn1nnc(c1)COc1nn2c(nnc2c2c1cccc2)c1noc(c1)C
UNII:
1M7NI1A92L
Properties
Complexity:
527
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
362.124g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
362.353g/mol
Monoisotopic Mass:
362.124g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
109A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Specific targeting of the GABA-A receptor α5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. | Journal of psychopharmacology (Oxford, England) 20110801 |
| Chronic Treatment with a Promnesiant GABA-A α5-Selective Inverse Agonist Increases Immediate Early Genes Expression during Memory Processing in Mice and Rectifies Their Expression Levels in a Down Syndrome Mouse Model. | Advances in pharmacological sciences 20110101 |
| Occupancy of human brain GABA(A) receptors by the novel α5 subtype-selective benzodiazepine site inverse agonist α5IA as measured using [¹¹C]flumazenil PET imaging. | Neuropharmacology 20101201 |
| The plasma-occupancy relationship of the novel GABAA receptor benzodiazepine site ligand, alpha5IA, is similar in rats and primates. | British journal of pharmacology 20090701 |
| An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. | Journal of medicinal chemistry 20041118 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







