200,000+ products from a single source!
sales@angenechem.com
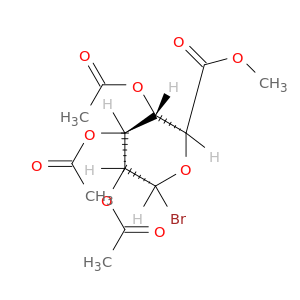
21085-72-3 | Methyl acetobromo-alpha-D-glucuronate
CAS No: 21085-72-3 Catalog No: AG00385Z MDL No:MFCD00061613
Product Description
Catalog Number:
AG00385Z
Chemical Name:
Methyl acetobromo-alpha-D-glucuronate
CAS Number:
21085-72-3
Molecular Formula:
C13H17BrO9
Molecular Weight:
397.1727
MDL Number:
MFCD00061613
IUPAC Name:
methyl (2S,3S,4S,5R,6R)-3,4,5-triacetyloxy-6-bromooxane-2-carboxylate
InChI:
InChI=1S/C13H17BrO9/c1-5(15)20-8-9(21-6(2)16)11(13(18)19-4)23-12(14)10(8)22-7(3)17/h8-12H,1-4H3/t8-,9-,10+,11-,12-/m0/s1
InChI Key:
GWTNLHGTLIBHHZ-SVNGYHJRSA-N
SMILES:
COC(=O)[C@H]1O[C@H](Br)[C@@H]([C@H]([C@@H]1OC(=O)C)OC(=O)C)OC(=O)C
EC Number:
244-203-4
Properties
Complexity:
492
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
396.006g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
9
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
397.174g/mol
Monoisotopic Mass:
396.006g/mol
Rotatable Bond Count:
8
Topological Polar Surface Area:
114A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.6
Literature
| Title | Journal |
|---|---|
| An improved chemo-enzymatic synthesis of 1-beta-O-acyl glucuronides: highly chemoselective enzymatic removal of protecting groups from corresponding methyl acetyl derivatives. | The Journal of organic chemistry 20071207 |
| Synthesis of 1-beta-O-acyl glucuronides of diclofenac, mefenamic acid and (S)-naproxen by the chemo-selective enzymatic removal of protecting groups from the corresponding methyl acetyl derivatives. | Organic & biomolecular chemistry 20060907 |
| Preparation of pyridine-N-glucuronides of tobacco-specific nitrosamines. | Chemical research in toxicology 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.