200,000+ products from a single source!
sales@angenechem.com
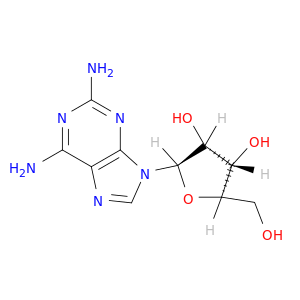
2096-10-8 | Adenosine, 2-amino-
CAS No: 2096-10-8 Catalog No: AG002KBV MDL No:MFCD00053556
Product Description
Catalog Number:
AG002KBV
Chemical Name:
Adenosine, 2-amino-
CAS Number:
2096-10-8
Molecular Formula:
C10H14N6O4
Molecular Weight:
282.2560
MDL Number:
MFCD00053556
IUPAC Name:
(2R,3R,4S,5R)-2-(2,6-diaminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
InChI:
InChI=1S/C10H14N6O4/c11-7-4-8(15-10(12)14-7)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H4,11,12,14,15)/t3-,5-,6-,9-/m1/s1
InChI Key:
ZDTFMPXQUSBYRL-UUOKFMHZSA-N
SMILES:
OC[C@H]1O[C@H]([C@@H]([C@@H]1O)O)n1cnc2c1nc(N)nc2N
Properties
Complexity:
363
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
282.108g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
9
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
282.26g/mol
Monoisotopic Mass:
282.108g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
166A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.4
Literature
| Title | Journal |
|---|---|
| S-Adenosylhomocysteine hydrolase of the protozoan parasite Trichomonas vaginalis: potent inhibitory activity of 9-(2-deoxy-2-fluoro-β,D-arabinofuranosyl)adenine. | Bioorganic & medicinal chemistry letters 20120615 |
| Adenosine deaminase-like protein 1 (ADAL1): characterization and substrate specificity in the hydrolysis of N(6)- or O(6)-substituted purine or 2-aminopurine nucleoside monophosphates. | Journal of medicinal chemistry 20110825 |
| An efficient and scalable synthesis of 2,6-diaminopurine riboside. | Nucleosides, nucleotides & nucleic acids 20080101 |
| Pyrazolo[3,4-d]pyrimidine ribonucleosides related to 2-aminoadenosine and isoguanosine: synthesis, deamination and tautomerism. | Organic & biomolecular chemistry 20070921 |
| Identification of an in vivo inhibitor of Bacillus anthracis spore germination. | The Journal of biological chemistry 20070420 |
| An efficient process for synthesis of 2'-O-methyl and 3'-O-methyl guanosine from 2-aminoadenosine using diazomethane and the catalyst stannous chloride. | Nucleosides, nucleotides & nucleic acids 20060301 |
| Quantitative assessment of the use of modified nucleoside triphosphates in expression profiling: differential effects on signal intensities and impacts on expression ratios. | BMC biotechnology 20020101 |
| Chemical constituents of Typhonium giganteum Engl. | Journal of Asian natural products research 20010101 |
| Acyclic nucleotide analogues: synthesis, antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. | Antiviral research 19900601 |
| Synthesis and antiviral activity of carbocyclic analogues of 2'-deoxyribofuranosides of 2-amino-6-substituted-purines and of 2-amino-6-substituted-8-azapurines. | Journal of medicinal chemistry 19841101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.