200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 20578-92-1
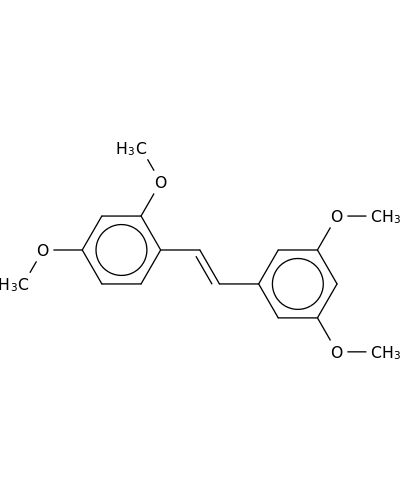
20578-92-1 | Benzene, 1-[2-(3,5-dimethoxyphenyl)ethenyl]-2,4-dimethoxy-
CAS No: 20578-92-1 Catalog No: AG002ACH MDL No:
Product Description
Catalog Number:
AG002ACH
Chemical Name:
Benzene, 1-[2-(3,5-dimethoxyphenyl)ethenyl]-2,4-dimethoxy-
CAS Number:
20578-92-1
Molecular Formula:
C18H20O4
Molecular Weight:
300.3490
IUPAC Name:
1-[(E)-2-(2,4-dimethoxyphenyl)ethenyl]-3,5-dimethoxybenzene
InChI:
InChI=1S/C18H20O4/c1-19-15-8-7-14(18(12-15)22-4)6-5-13-9-16(20-2)11-17(10-13)21-3/h5-12H,1-4H3/b6-5+
InChI Key:
JDBCWSHYEQUBLW-AATRIKPKSA-N
SMILES:
COc1ccc(c(c1)OC)C=Cc1cc(OC)cc(c1)OC
Properties
Complexity:
332
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
300.136g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
300.354g/mol
Monoisotopic Mass:
300.136g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
36.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.1
Literature
| Title | Journal |
|---|---|
| Hydroxystilbenes and methoxystilbenes activate human aryl hydrocarbon receptor and induce CYP1A genes in human hepatoma cells and human hepatocytes. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20170501 |
| Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. | Circulation 20120828 |
| Involvement of cytochrome P-450 1B1 in renal dysfunction, injury, and inflammation associated with angiotensin II-induced hypertension in rats. | American journal of physiology. Renal physiology 20120215 |
| CYP1B1 detection. | Current protocols in toxicology 20120201 |
| The resveratrol analogue, 2,3',4,5'-tetramethoxystilbene, does not inhibit CYP gene expression, enzyme activity and benzo[a]pyrene-DNA adduct formation in MCF-7 cells exposed to benzo[a]pyrene. | Mutagenesis 20110901 |
| Tetra-methoxystilbene modulates ductal growth of the developing murine mammary gland. | Breast cancer research and treatment 20110401 |
| 2,3',4,5'-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: contribution of cytochrome P-450 1B1. | American journal of physiology. Heart and circulatory physiology 20101201 |
| Quantification of oxyresveratrol analog trans-2,4,3',5'-tetramethoxystilbene in rat plasma by a rapid HPLC method: application in a pre-clinical pharmacokinetic study. | Biomedical chromatography : BMC 20101201 |
| TMS, a chemically modified herbal derivative of resveratrol, induces cell death by targeting Bax. | Breast cancer research and treatment 20101101 |
| Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. | Hypertension (Dallas, Tex. : 1979) 20101001 |
| Reverse type I binding spectra of human cytochrome P450 1B1 induced by flavonoid, stilbene, pyrene, naphthalene, phenanthrene, and biphenyl derivatives that inhibit catalytic activity: a structure-function relationship study. | Chemical research in toxicology 20090701 |
| Induction of p27(kip1) by 2,4,3',5'- tetramethoxystilbene is regulated by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. | Archives of pharmacal research 20080901 |
| Trans- and cis-stilbene polyphenols induced rapid perinuclear mitochondrial clustering and p53-independent apoptosis in cancer cells but not normal cells. | European journal of pharmacology 20080610 |
| DNA adducts formation and induction of apoptosis in rat liver epithelial 'stem-like' cells exposed to carcinogenic polycyclic aromatic hydrocarbons. | Mutation research 20080201 |
| Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. | Molecular pharmacology 20071101 |
| Regioselective 2-hydroxylation of 17beta-estradiol by rat cytochrome P4501B1. | Toxicology and applied pharmacology 20061101 |
| Chemical transformations of oxyresveratrol (trans-2,4,3',5'-tetrahydroxystilbene) into a potent tyrosinase inhibitor and a strong cytotoxic agent. | Bioorganic & medicinal chemistry letters 20061101 |
| Modulation of human cytochrome P450 1B1 expression by 2,4,3',5'-tetramethoxystilbene. | Drug metabolism and disposition: the biological fate of chemicals 20051201 |
| Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. | The Journal of biological chemistry 20051111 |
| Metabolism of melatonin by human cytochromes p450. | Drug metabolism and disposition: the biological fate of chemicals 20050401 |
| Reduction of androgen receptor expression by benzo[alpha]pyrene and 7,8-dihydro-9,10-epoxy-7,8,9,10-tetrahydrobenzo[alpha]pyrene in human lung cells. | Biochemical pharmacology 20040415 |
| Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. | Journal of medicinal chemistry 20020103 |
| A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. | Cancer research 20011115 |
| Resveratrol analog, 3,5,2',4'-tetramethoxy-trans-stilbene, potentiates the inhibition of cell growth and induces apoptosis in human cancer cells. | Archives of pharmacal research 20011001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.