200,000+ products from a single source!
sales@angenechem.com
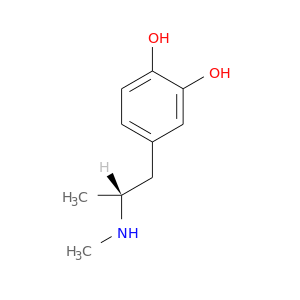
20521-19-1 | 1,2-Benzenediol, 4-[(2R)-2-(methylamino)propyl]-
CAS No: 20521-19-1 Catalog No: AG0029YO MDL No:
Product Description
Catalog Number:
AG0029YO
Chemical Name:
1,2-Benzenediol, 4-[(2R)-2-(methylamino)propyl]-
CAS Number:
20521-19-1
Molecular Formula:
C10H15NO2
Molecular Weight:
181.2316
IUPAC Name:
4-[(2R)-2-(methylamino)propyl]benzene-1,2-diol
InChI:
InChI=1S/C10H15NO2/c1-7(11-2)5-8-3-4-9(12)10(13)6-8/h3-4,6-7,11-13H,5H2,1-2H3/t7-/m1/s1
InChI Key:
NTCPGTZTPGFNOM-SSDOTTSWSA-N
SMILES:
CN[C@@H](Cc1ccc(c(c1)O)O)C
Properties
Complexity:
152
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
181.11g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
181.235g/mol
Monoisotopic Mass:
181.11g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
52.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0
Literature
| Title | Journal |
|---|---|
| Duloxetine inhibits effects of MDMA ('ecstasy') in vitro and in humans in a randomized placebo-controlled laboratory study. | PloS one 20120101 |
| Induction of glutathione synthesis and conjugation by 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-dihydroxymethamphetamine (HHMA) in human and rat liver cells, including the protective role of some antioxidants. | Toxicology 20111118 |
| Investigation on the enantioselectivity of the sulfation of the methylenedioxymethamphetamine metabolites 3,4-dihydroxymethamphetamine and 4-hydroxy-3-methoxymethamphetamine using the substrate-depletion approach. | Drug metabolism and disposition: the biological fate of chemicals 20111101 |
| Inhibition of 3,4-methylenedioxymethamphetamine metabolism leads to marked decrease in 3,4-dihydroxymethamphetamine formation but no change in serotonin neurotoxicity: implications for mechanisms of neurotoxicity. | Synapse (New York, N.Y.) 20111001 |
| Metabolism and disposition of 3,4-methylenedioxymethamphetamine ('ecstasy') in baboons after oral administration: comparison with humans reveals marked differences. | The Journal of pharmacology and experimental therapeutics 20110701 |
| Development and validation of LC-HRMS and GC-NICI-MS methods for stereoselective determination of MDMA and its phase I and II metabolites in human urine. | Journal of mass spectrometry : JMS 20110701 |
| Sulfation of the 3,4-methylenedioxymethamphetamine (MDMA) metabolites 3,4-dihydroxymethamphetamine (DHMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) and their capability to inhibit human sulfotransferases. | Toxicology letters 20110425 |
| Synthesis and in vitro cytotoxicity profile of the R-enantiomer of 3,4-dihydroxymethamphetamine (R-(-)-HHMA): comparison with related catecholamines. | Chemical research in toxicology 20100101 |
| Effect of the CB1 cannabinoid agonist WIN 55212-2 on the acquisition and reinstatement of MDMA-induced conditioned place preference in mice. | Behavioral and brain functions : BBF 20100101 |
| Further studies on the role of metabolites in (+/-)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. | Drug metabolism and disposition: the biological fate of chemicals 20091001 |
| Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. | Drug metabolism and disposition: the biological fate of chemicals 20090701 |
| Direct comparison of (+/-) 3,4-methylenedioxymethamphetamine ('ecstasy') disposition and metabolism in squirrel monkeys and humans. | Therapeutic drug monitoring 20090601 |
| Hydrolysis of 3,4-methylenedioxymethamphetamine (MDMA) metabolite conjugates in human, squirrel monkey, and rat plasma. | Analytical and bioanalytical chemistry 20090301 |
| [Metabolites of ecstasy and cytotoxicity effects]. | Annales pharmaceutiques francaises 20090301 |
| Simultaneous liquid chromatographic-electrospray ionization mass spectrometric quantification of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its metabolites 3,4-dihydroxymethamphetamine, 4-hydroxy-3-methoxymethamphetamine and 3,4-methylenedioxyamphetamine in squirrel monkey and human plasma after acidic conjugate cleavage. | Forensic science international 20090130 |
| Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy'): synthesis, isolation, and characterization of diastereoisomers. | Chemical research in toxicology 20081201 |
| Nonlinear pharmacokinetics of (+/-)3,4-methylenedioxymethamphetamine (MDMA, 'Ecstasy') and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. | The Journal of pharmacology and experimental therapeutics 20081001 |
| Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. | Drug metabolism and disposition: the biological fate of chemicals 20071001 |
| Validated liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in squirrel monkey plasma. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20070815 |
| 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. | Psychopharmacology 20070101 |
| A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. | British journal of pharmacology 20050101 |
| Contribution of cytochrome P450 2D6 to 3,4-methylenedioxymethamphetamine disposition in humans: use of paroxetine as a metabolic inhibitor probe. | Clinical pharmacokinetics 20050101 |
| Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites in human samples including the catechol-type metabolite (3,4-dihydroxymethamphetamine). | Drug metabolism and disposition: the biological fate of chemicals 20040901 |
| Synthesis, in vitro formation, and behavioural effects of glutathione regioisomers of alpha-methyldopamine with relevance to MDA and MDMA (ecstasy). | Brain research 20031017 |
| Action of MDMA (ecstasy) and its metabolites on arginine vasopressin release. | Annals of the New York Academy of Sciences 20020601 |
| High-performance liquid chromatography with electrochemical detection applied to the analysis of 3,4-dihydroxymethamphetamine in human plasma and urine. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20020405 |
| 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. | Chemical research in toxicology 20010901 |
Related Products
© 2019 Angene International Limited. All rights Reserved.