200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 2044-64-6
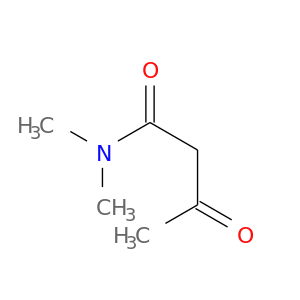
2044-64-6 | Butanamide, N,N-dimethyl-3-oxo-
CAS No: 2044-64-6 Catalog No: AG0029HG MDL No:MFCD00038243
Product Description
Catalog Number:
AG0029HG
Chemical Name:
Butanamide, N,N-dimethyl-3-oxo-
CAS Number:
2044-64-6
Molecular Formula:
C6H11NO2
Molecular Weight:
129.1570
MDL Number:
MFCD00038243
IUPAC Name:
N,N-dimethyl-3-oxobutanamide
InChI:
InChI=1S/C6H11NO2/c1-5(8)4-6(9)7(2)3/h4H2,1-3H3
InChI Key:
YPEWWOUWRRQBAX-UHFFFAOYSA-N
SMILES:
CC(=O)CC(=O)N(C)C
EC Number:
218-059-8
UNII:
7E2L0OAA4K
NSC Number:
524755
Properties
Complexity:
129
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
129.079g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
129.159g/mol
Monoisotopic Mass:
129.079g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
37.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.4
Literature
| Title | Journal |
|---|---|
| Disposition and metabolism of N,N-dimethylacetoacetamide in male F344 and Wistar-Han rats and female B6C3F1 mice. | Xenobiotica; the fate of foreign compounds in biological systems 20111101 |
| Free radical reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds. | Organic & biomolecular chemistry 20091007 |
| Reduction of ketones and alkyl iodides by SmI(2) and Sm(II)-HMPA complexes. Rate and mechanistic studies. | Journal of the American Chemical Society 20020619 |
| Mechanistic study of beta-substituent effects on the mechanism of ketone reduction by SmI(2). | Journal of the American Chemical Society 20020605 |
Related Products
© 2019 Angene International Limited. All rights Reserved.