200,000+ products from a single source!
sales@angenechem.com
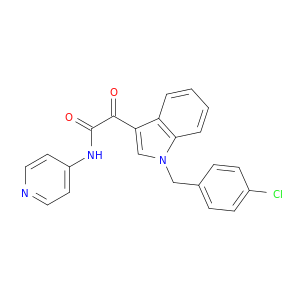
204205-90-3 | 1H-Indole-3-acetamide, 1-[(4-chlorophenyl)methyl]-α-oxo-N-4-pyridinyl-
CAS No: 204205-90-3 Catalog No: AG0029CB MDL No:MFCD05861105
Product Description
Catalog Number:
AG0029CB
Chemical Name:
1H-Indole-3-acetamide, 1-[(4-chlorophenyl)methyl]-α-oxo-N-4-pyridinyl-
CAS Number:
204205-90-3
Molecular Formula:
C22H16ClN3O2
Molecular Weight:
389.8343
MDL Number:
MFCD05861105
IUPAC Name:
2-[1-[(4-chlorophenyl)methyl]indol-3-yl]-2-oxo-N-pyridin-4-ylacetamide
InChI:
InChI=1S/C22H16ClN3O2/c23-16-7-5-15(6-8-16)13-26-14-19(18-3-1-2-4-20(18)26)21(27)22(28)25-17-9-11-24-12-10-17/h1-12,14H,13H2,(H,24,25,28)
InChI Key:
SOLIIYNRSAWTSQ-UHFFFAOYSA-N
SMILES:
Clc1ccc(cc1)Cn1cc(c2c1cccc2)C(=O)C(=O)Nc1ccncc1
UNII:
80K4H2RB8P
Properties
Complexity:
558
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
389.093g/mol
Formal Charge:
0
Heavy Atom Count:
28
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
389.839g/mol
Monoisotopic Mass:
389.093g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
64A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| [Molecular dynamics simulation of the tubulin dimer with cytostatics]. | Molekuliarnaia biologiia 20120101 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20101201 |
| Dose-finding and pharmacokinetic study of orally administered indibulin (D-24851) to patients with advanced solid tumors. | Investigational new drugs 20100401 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20100101 |
| Indolobenzazepin-7-ones and 6-, 8-, and 9-membered ring derivatives as tubulin polymerization inhibitors: synthesis and structure--activity relationship studies. | Journal of medicinal chemistry 20091008 |
| Synthesis and structure-activity relationships of N-aryl(indol-3-yl)glyoxamides as antitumor agents. | Bioorganic & medicinal chemistry 20090915 |
| Indibulin, a novel microtubule inhibitor, discriminates between mature neuronal and nonneuronal tubulin. | Cancer research 20090101 |
| Side chain modifications of (indol-3-yl)glyoxamides as antitumor agents. | Journal of enzyme inhibition and medicinal chemistry 20081001 |
| Design, synthesis and cytotoxicity of novel podophyllotoxin derivatives. | Chemical & pharmaceutical bulletin 20080601 |
| Indole- and indolizine-glyoxylamides displaying cytotoxicity against multidrug resistant cancer cell lines. | Bioorganic & medicinal chemistry letters 20080315 |
| In vivo evaluation of indolyl glyoxamides in the phenotypic sea urchin embryo assay. | Chemical biology & drug design 20071201 |
| Phase I dose-finding and pharmacokinetic trial of orally administered indibulin (D-24851) to patients with solid tumors. | Investigational new drugs 20070601 |
| Antirestenotic effects of a novel polymer-coated d-24851 eluting stent. Experimental data in a rabbit iliac artery model. | Cardiovascular and interventional radiology 20070101 |
| Microtubule inhibitor D-24851 induces p53-independent apoptotic cell death in malignant glioma cells through Bcl-2 phosphorylation and Bax translocation. | International journal of oncology 20050301 |
| Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? | Rapid communications in mass spectrometry : RCM 20050101 |
| Quantitative analysis of D-24851, a novel anticancer agent, in human plasma and urine by liquid chromatography coupled with tandem mass spectrometry. | Rapid communications in mass spectrometry : RCM 20040101 |
| Differential roles of p21(Waf1) and p27(Kip1) in modulating chemosensitivity and their possible application in drug discovery studies. | Molecular pharmacology 20011101 |
| D-24851, a novel synthetic microtubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy toward multidrug-resistant tumor cells, and lacks neurotoxicity. | Cancer research 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.