200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 203923-89-1
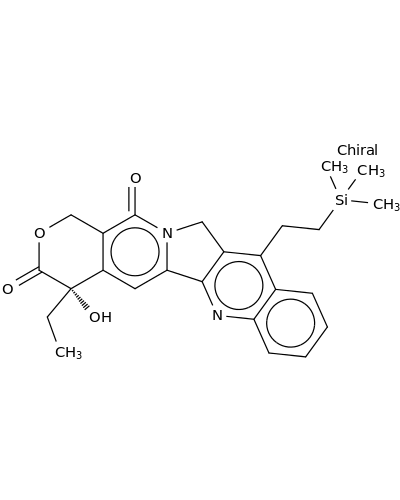
203923-89-1 | 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-11-[2-(trimethylsilyl)ethyl]-, (4S)-
CAS No: 203923-89-1 Catalog No: AG00297R MDL No:
Product Description
Catalog Number:
AG00297R
Chemical Name:
1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-11-[2-(trimethylsilyl)ethyl]-, (4S)-
CAS Number:
203923-89-1
Molecular Formula:
C25H28N2O4Si
Molecular Weight:
448.5863
IUPAC Name:
(19S)-19-ethyl-19-hydroxy-10-(2-trimethylsilylethyl)-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaene-14,18-dione
InChI:
InChI=1S/C25H28N2O4Si/c1-5-25(30)19-12-21-22-17(13-27(21)23(28)18(19)14-31-24(25)29)15(10-11-32(2,3)4)16-8-6-7-9-20(16)26-22/h6-9,12,30H,5,10-11,13-14H2,1-4H3/t25-/m0/s1
InChI Key:
POADTFBBIXOWFJ-VWLOTQADSA-N
SMILES:
CC[C@@]1(O)C(=O)OCc2c1cc1c3nc4ccccc4c(c3Cn1c2=O)CC[Si](C)(C)C
UNII:
24R60NVC41
NSC Number:
710270
Properties
Complexity:
891
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
448.182g/mol
Formal Charge:
0
Heavy Atom Count:
32
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
448.594g/mol
Monoisotopic Mass:
448.182g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
79.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Preclinical and clinical activity of the topoisomerase I inhibitor, karenitecin, in melanoma. | Expert opinion on investigational drugs 20111101 |
| Alveolar rhabdomyosarcoma of the extremity and nodal metastasis: Is the in-transit lymphatic system at risk? | Pediatric blood & cancer 20091215 |
| Organic anion-transporting polypeptide 1B1 mediates transport of Gimatecan and BNP1350 and can be inhibited by several classic ATP-binding cassette (ABC) B1 and/or ABCG2 inhibitors. | Drug metabolism and disposition: the biological fate of chemicals 20090401 |
| Potentiation of a topoisomerase I inhibitor, karenitecin, by the histone deacetylase inhibitor valproic acid in melanoma: translational and phase I/II clinical trial. | Clinical cancer research : an official journal of the American Association for Cancer Research 20090401 |
| Phase I and pharmacokinetic study of karenitecin in patients with recurrent malignant gliomas. | Neuro-oncology 20080801 |
| Synergy of karenitecin and mafosfamide in pediatric leukemia, medulloblastoma, and neuroblastoma cell lines. | Pediatric blood & cancer 20080401 |
| Phase II multicenter open-label study of karenitecin in previously treated epithelial ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. | International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 20080101 |
| Phase II trial of karenitecin in patients with relapsed or refractory non-small cell lung cancer (CALGB 30004). | Lung cancer (Amsterdam, Netherlands) 20050601 |
| Phase II trial of karenitecin in patients with malignant melanoma: clinical and translational study. | Clinical cancer research : an official journal of the American Association for Cancer Research 20050415 |
| Plasma and cerebrospinal fluid pharmacokinetic study of BNP1350 in nonhuman primates. | Cancer chemotherapy and pharmacology 20040601 |
| Evaluation of in vitro drug interactions with karenitecin, a novel, highly lipophilic camptothecin derivative in phase II clinical development. | Journal of clinical pharmacology 20030901 |
| [A symphony for the camptothecins]. | Bulletin du cancer 20030301 |
| Chk1 signaling pathways that mediated G(2)M checkpoint in relation to the cellular resistance to the novel topoisomerase I poison BNP1350. | Biochemical and biophysical research communications 20020712 |
| Novel camptothecin derivative BNP1350 in experimental human ovarian cancer: determination of efficacy and possible mechanisms of resistance. | International journal of cancer 20020701 |
| Development of a high-performance liquid chromatographic method to determine the concentration of karenitecin, a novel highly lipophilic camptothecin derivative, in human plasma and urine. | Journal of chromatography. B, Biomedical sciences and applications 20010805 |
| Therapeutic activity of 7-[(2-trimethylsilyl)ethyl)]-20 (S)-camptothecin against central nervous system tumor-derived xenografts in athymic mice. | Cancer chemotherapy and pharmacology 20010701 |
| New highly lipophilic camptothecin BNP1350 is an effective drug in experimental human cancer. | International journal of cancer 20001015 |
| Characterization of protein kinase chk1 essential for the cell cycle checkpoint after exposure of human head and neck carcinoma A253 cells to a novel topoisomerase I inhibitor BNP1350. | Molecular pharmacology 20000301 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.