200,000+ products from a single source!
sales@angenechem.com
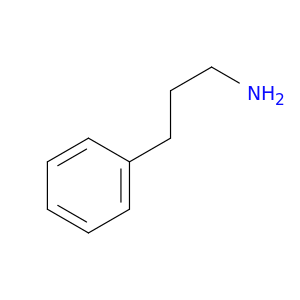
2038-57-5 | Benzenepropanamine
CAS No: 2038-57-5 Catalog No: AG00294J MDL No:MFCD00008224
Product Description
Catalog Number:
AG00294J
Chemical Name:
Benzenepropanamine
CAS Number:
2038-57-5
Molecular Formula:
C9H13N
Molecular Weight:
135.2062
MDL Number:
MFCD00008224
IUPAC Name:
3-phenylpropan-1-amine
InChI:
InChI=1S/C9H13N/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8,10H2
InChI Key:
LYUQWQRTDLVQGA-UHFFFAOYSA-N
SMILES:
NCCCc1ccccc1
EC Number:
218-012-1
UNII:
P8326EZ31P
NSC Number:
87080
Properties
Complexity:
74.8
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
135.105g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
135.21g/mol
Monoisotopic Mass:
135.105g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
26A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.8
Literature
| Title | Journal |
|---|---|
| Benzaldehyde is a precursor of phenylpropylamino alkaloids as revealed by targeted metabolic profiling and comparative biochemical analyses in Ephedra spp. | Phytochemistry 20120901 |
| Naturally occurring norephedrine oxazolidine derivatives in khat (Catha edulis). | Planta medica 20120501 |
| Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. | Emergency medicine journal : EMJ 20110801 |
| Comparative Molecular Field Analysis (CoMFA) and Comparative Molecular Similarity Indices Analysis (CoMSIA) studies on α(1A)-adrenergic receptor antagonists based on pharmacophore molecular alignment. | International journal of molecular sciences 20110101 |
| Expressed sequence tag analysis of khat (Catha edulis) provides a putative molecular biochemical basis for the biosynthesis of phenylpropylamino alkaloids. | Genetics and molecular biology 20110101 |
| Experimental and computational studies of enantioseparation of structurally similar chiral compounds on amylose tris(3,5-dimethylphenylcarbamate). | Chirality 20100601 |
| Composition and stereochemistry of ephedrine alkaloids accumulation in Ephedra sinica Stapf. | Phytochemistry 20100601 |
| Rapid, sensitive and simultaneous determination of fluorescence-labeled designated substances controlled by the Pharmaceutical Affairs Law in Japan by ultra-performance liquid chromatography coupled with electrospray-ionization time-of-flight mass spectrometry. | Analytical and bioanalytical chemistry 20091101 |
| The hydrogen-bonding patterns of 3-phenylpropylammonium benzoate and 3-phenylpropylammonium 3-iodobenzoate: generation of chiral crystals from achiral molecules. | Acta crystallographica. Section C, Crystal structure communications 20081201 |
| Selective extraction and elution of weak bases by in-line solid-phase extraction capillary electrophoresis using a pH step gradient and a weak cation-exchange monolith. | The Analyst 20081001 |
| Developmental patterns of phenylpropylamino alkaloids accumulation in khat (Catha edulis, Forsk.). | Journal of ethnopharmacology 20071203 |
| New efficient substrates for semicarbazide-sensitive amine oxidase/VAP-1 enzyme: analysis by SARs and computational docking. | Journal of medicinal chemistry 20061019 |
| Synthesis of benzenepropanamine analogues as non-detergent spermicides with antitrichomonas and anticandida activities. | Bioorganic & medicinal chemistry 20061001 |
| Aromatic chlorination of omega-phenylalkylamines and omega-phenylalkylamides in carbon tetrachloride and alpha,alpha,alpha-trifluorotoluene. | Organic & biomolecular chemistry 20060721 |
| catena-Poly[tetrakis(3-phenylpropylammonium) [iodoplumbate(II)-tri-mu-iodo-plumbate(II)-tri-mu-iodo-iodoplumbate(II)-di-mu-iodo]]. | Acta crystallographica. Section C, Crystal structure communications 20060501 |
| N-benzylethylammonium nitrate: a three-dimensional hydrogen-bonded framework comprising substructures in zero, one and two dimensions. | Acta crystallographica. Section C, Crystal structure communications 20051101 |
| Simple, intuitive calculations of free energy of binding for protein-ligand complexes. 1. Models without explicit constrained water. | Journal of medicinal chemistry 20020606 |
| N-arylalkylpiperidines as high-affinity sigma-1 and sigma-2 receptor ligands: phenylpropylamines as potential leads for selective sigma-2 agents. | Bioorganic & medicinal chemistry letters 20020211 |
Related Products
© 2019 Angene International Limited. All rights Reserved.