200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 20290-99-7
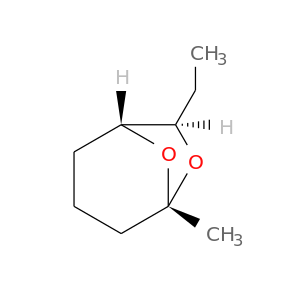
20290-99-7 | 6,8-Dioxabicyclo[3.2.1]octane, 7-ethyl-5-methyl-, (1R,5S,7R)-
CAS No: 20290-99-7 Catalog No: AG0028J1 MDL No:
Product Description
Catalog Number:
AG0028J1
Chemical Name:
6,8-Dioxabicyclo[3.2.1]octane, 7-ethyl-5-methyl-, (1R,5S,7R)-
CAS Number:
20290-99-7
Molecular Formula:
C9H16O2
Molecular Weight:
156.2221
IUPAC Name:
(1R,5S,7R)-7-ethyl-5-methyl-6,8-dioxabicyclo[3.2.1]octane
InChI:
InChI=1S/C9H16O2/c1-3-7-8-5-4-6-9(2,10-7)11-8/h7-8H,3-6H2,1-2H3/t7-,8-,9+/m1/s1
InChI Key:
YONXEBYXWVCXIV-HLTSFMKQSA-N
SMILES:
CC[C@H]1O[C@]2(O[C@@H]1CCC2)C
UNII:
KPU1SW45CD
Properties
Complexity:
158
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
156.115g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
156.225g/mol
Monoisotopic Mass:
156.115g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
18.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| Variable responses by southern pine beetle, Dendroctonus frontalis Zimmermann, to the pheromone component endo-brevicomin: influence of enantiomeric composition, release rate, and proximity to infestations. | Journal of chemical ecology 20110401 |
| Protective group-free syntheses of (±)-frontalin, (±)-endo-brevicomin, (±)-exo-brevicomin, and (±)-3,4-dehydro-exo-brevicomin: racemic pheromones with a 6,8-dioxabicyclo[3.2.1]octane ring. | Bioscience, biotechnology, and biochemistry 20110101 |
| Impacts of silvicultural thinning treatments on beetle trap captures and tree attacks during low bark beetle populations in ponderosa pine forests of northern Arizona. | Journal of economic entomology 20101001 |
| Spatial displacement of release point can enhance activity of an attractant pheromone synergist of a bark beetle. | Journal of chemical ecology 20091001 |
| Organometallic enantiomeric scaffolding. A molybdenum-mediated intramolecular nucleophilic ketalization-demetalation cascade. Total synthesis of (+)-(1R,2S,5S,7R)-2-hydroxy-exo-brevicomin. | Organic letters 20090820 |
| Electrophysiological and behavioral responses of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae) to four bark beetle pheromones. | Environmental entomology 20090401 |
| Field response of Dendroctonus frontalis (Coleoptera: Scolytinae) to synthetic semiochemicals in Chiapas, Mexico. | Journal of economic entomology 20081201 |
| Mice recognize recent urine scent marks by the molecular composition. | Chemical senses 20080901 |
| Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). | Journal of economic entomology 20080801 |
| Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. | Environmental entomology 20080201 |
| High individual variation in pheromone production by tree-killing bark beetles (Coleoptera: Curculionidae: Scolytinae). | Die Naturwissenschaften 20080101 |
| Evidence that (+)-endo-brevicomin is a male-produced component of the Southern pine beetle aggregation pheromone. | Journal of chemical ecology 20070801 |
| Insect pheromones and precursors in female African elephant urine. | Journal of chemical ecology 20060801 |
| Preparative chiral liquid chromatography for enantiomeric separation of pheromones. | Journal of chemical ecology 20010301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.