200,000+ products from a single source!
sales@angenechem.com
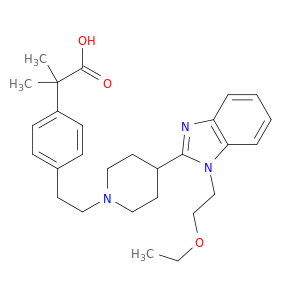
202189-78-4 | Benzeneacetic acid, 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl-
CAS No: 202189-78-4 Catalog No: AG002844 MDL No:MFCD09837814
Product Description
Catalog Number:
AG002844
Chemical Name:
Benzeneacetic acid, 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl-
CAS Number:
202189-78-4
Molecular Formula:
C28H37N3O3
Molecular Weight:
463.6117
MDL Number:
MFCD09837814
IUPAC Name:
2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylpropanoic acid
InChI:
InChI=1S/C28H37N3O3/c1-4-34-20-19-31-25-8-6-5-7-24(25)29-26(31)22-14-17-30(18-15-22)16-13-21-9-11-23(12-10-21)28(2,3)27(32)33/h5-12,22H,4,13-20H2,1-3H3,(H,32,33)
InChI Key:
ACCMWZWAEFYUGZ-UHFFFAOYSA-N
SMILES:
CCOCCn1c(nc2c1cccc2)C1CCN(CC1)CCc1ccc(cc1)C(C(=O)O)(C)C
UNII:
PA1123N395
Properties
Complexity:
641
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
463.283g/mol
Formal Charge:
0
Heavy Atom Count:
34
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
463.622g/mol
Monoisotopic Mass:
463.283g/mol
Rotatable Bond Count:
10
Topological Polar Surface Area:
67.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.3
Literature
| Title | Journal |
|---|---|
| Treatment of allergic rhinitis and urticaria: a review of the newest antihistamine drug bilastine. | Therapeutics and clinical risk management 20160101 |
| Bilastine: in allergic rhinitis and urticaria. | Drugs 20120618 |
| Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. | Journal of clinical pharmacology 20120601 |
| Whole-body tissue distribution of total radioactivity in rats after oral administration of [¹⁴C]-bilastine. | Drug and chemical toxicology 20120601 |
| Interactions of bilastine, a new oral H₁ antihistamine, with human transporter systems. | Drug and chemical toxicology 20120601 |
| An overview of bilastine metabolism during preclinical investigations. | Drug and chemical toxicology 20120601 |
| Preclinical toxicity profile of oral bilastine. | Drug and chemical toxicology 20120601 |
| Effects of bilastine on T-wave morphology and the QTc interval: a randomized, double-blind, placebo-controlled, thorough QTc study. | Clinical drug investigation 20120501 |
| Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitis. | Current medical research and opinion 20120101 |
| An overview of the novel H1-antihistamine bilastine in allergic rhinitis and urticaria. | Expert review of clinical immunology 20120101 |
| Establishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence review. | Current medical research and opinion 20120101 |
| Comparative inhibition by bilastine and cetirizine of histamine-induced wheal and flare responses in humans. | Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20111201 |
| Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. | Journal of psychopharmacology (Oxford, England) 20111101 |
| Safety and efficacy of bilastine: a new H(1)-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. | Expert opinion on drug safety 20110901 |
| Bilastine for the relief of allergy symptoms. | Drugs of today (Barcelona, Spain : 1998) 20110401 |
| Focusing into new challenges in allergic rhinitis and urticaria. Introduction. | Journal of investigational allergology & clinical immunology 20110101 |
| Effect of bilastine upon nasal obstruction. | Journal of investigational allergology & clinical immunology 20110101 |
| Bilastine and the central nervous system. | Journal of investigational allergology & clinical immunology 20110101 |
| Bilastine and quality of life. | Journal of investigational allergology & clinical immunology 20110101 |
| Effect of bilastine upon the ocular symptoms of allergic rhinoconjunctivitis. | Journal of investigational allergology & clinical immunology 20110101 |
| The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. | Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20100501 |
| Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo-controlled study. | Allergy 20100401 |
| Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: a randomized, double-blind, parallel-group study. | Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 20090901 |
| [Antihistamines in the treatment of allergic rhinitis--update 2008/2009]. | Otolaryngologia polska = The Polish otolaryngology 20090901 |
| Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients. | Allergy 20090101 |
| Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine. | Clinical pharmacokinetics 20090101 |
| Comparison of peripheral and central effects of single and repeated oral dose administrations of bilastine, a new H1 antihistamine: a dose-range study in healthy volunteers with hydroxyzine and placebo as control treatments. | Journal of clinical psychopharmacology 20081201 |
| In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. | Drugs in R&D 20060101 |
| Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: receptor selectivity and in vitro antihistaminic activity. | Drugs in R&D 20050101 |
| Matrix solid-phase dispersion technique for the determination of a new antiallergic drug, bilastine, in rat faeces. | Journal of chromatography. B, Biomedical sciences and applications 20010825 |
Related Products
© 2019 Angene International Limited. All rights Reserved.