200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 20031-21-4
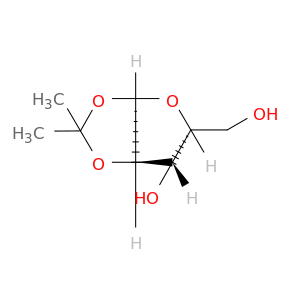
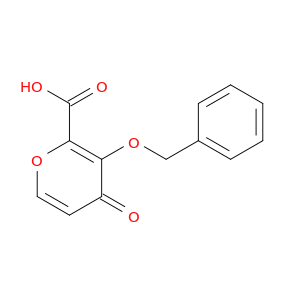
20031-21-4 | α-D-Xylofuranose, 1,2-O-(1-methylethylidene)-
CAS No: 20031-21-4 Catalog No: AG002COM MDL No:MFCD00063295
Product Description
Catalog Number:
AG002COM
Chemical Name:
α-D-Xylofuranose, 1,2-O-(1-methylethylidene)-
CAS Number:
20031-21-4
Molecular Formula:
C8H14O5
Molecular Weight:
190.1938
MDL Number:
MFCD00063295
IUPAC Name:
(3aR,5R,6S,6aR)-5-(hydroxymethyl)-2,2-dimethyl-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3]dioxol-6-ol
InChI:
InChI=1S/C8H14O5/c1-8(2)12-6-5(10)4(3-9)11-7(6)13-8/h4-7,9-10H,3H2,1-2H3/t4-,5+,6-,7-/m1/s1
InChI Key:
JAUQZVBVVJJRKM-XZBKPIIZSA-N
SMILES:
OC[C@H]1O[C@H]2[C@@H]([C@H]1O)OC(O2)(C)C
Properties
Complexity:
205
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
190.084g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
190.195g/mol
Monoisotopic Mass:
190.084g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
68.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.4
Literature
| Title | Journal |
|---|---|
| Efficient synthesis, structural characterization and anti-microbial activity of chiral aryl boronate esters of 1,2-O-isopropylidene-α-D-xylofuranose. | Bioorganic & medicinal chemistry letters 20110701 |
| A short and practical synthesis of two Hagen's gland lactones. | Carbohydrate research 20090612 |
| Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha. | Journal of medicinal chemistry 20081225 |
| Synthesis of highly condensed polycyclic carbohydrates by reaction of a spirocyclic enamino sulfonate derived from d-xylofuranose with bifunctional reagents. | The Journal of organic chemistry 20071207 |
| A cyclic enamine derived from 1,2-O-isopropylidene-alpha-D-xylofuranose as a novel carbohydrate intermediate to achieve skeletal diversity. | The Journal of organic chemistry 20060915 |
Related Products
© 2019 Angene International Limited. All rights Reserved.