200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 196929-78-9
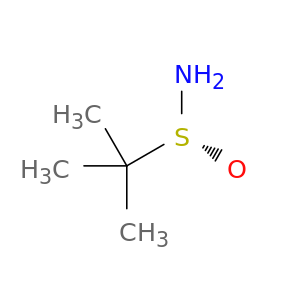
196929-78-9 | (R)-(+)-2-Methyl-2-Propanesulfinamide
CAS No: 196929-78-9 Catalog No: AG0032E1 MDL No:MFCD01863616
Product Description
Catalog Number:
AG0032E1
Chemical Name:
(R)-(+)-2-Methyl-2-Propanesulfinamide
CAS Number:
196929-78-9
Molecular Formula:
C4H11NOS
Molecular Weight:
121.2012
MDL Number:
MFCD01863616
IUPAC Name:
(R)-2-methylpropane-2-sulfinamide
InChI:
InChI=1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m1/s1
InChI Key:
CESUXLKAADQNTB-SSDOTTSWSA-N
SMILES:
CC([S@@](=O)N)(C)C
EC Number:
676-338-3
Properties
Complexity:
84.2
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
121.056g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
121.198g/mol
Monoisotopic Mass:
121.056g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
62.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0
Literature
| Title | Journal |
|---|---|
| (R)-N-(3-Meth-oxy-phen-yl)-tert-butane-sulfinamide. | Acta crystallographica. Section E, Structure reports online 20120301 |
| A novel asymmetric synthesis of cinacalcet hydrochloride. | Beilstein journal of organic chemistry 20120101 |
| Stereoselective synthesis of novel uracil polyoxin C conjugates as substrate analogues of chitin synthase. | The Journal of organic chemistry 20080516 |
| Synthesis of enantiopure tert-butanesulfinamide from tert-butanesulfinyloxazolidinone. | The Journal of organic chemistry 20041126 |
| Properly designed modular asymmetric synthesis for enantiopure sulfinamide auxiliaries from N-sulfonyl-1,2,3-oxathiazolidine-2-oxide agents. | Journal of the American Chemical Society 20020710 |
Related Products
© 2019 Angene International Limited. All rights Reserved.