200,000+ products from a single source!
sales@angenechem.com
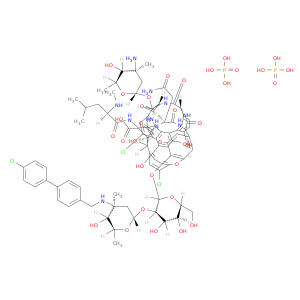
192564-14-0 | Vancomycin, 22-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-L-arabino-hexopyranosyl)-N3''-[(4'-chloro[1,1'-biphenyl]-4-yl)methyl]-, (4''R)-, phosphate (1:2)
CAS No: 192564-14-0 Catalog No: AG002G3Q MDL No:
Product Description
Catalog Number:
AG002G3Q
Chemical Name:
Vancomycin, 22-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-L-arabino-hexopyranosyl)-N3''-[(4'-chloro[1,1'-biphenyl]-4-yl)methyl]-, (4''R)-, phosphate (1:2)
CAS Number:
192564-14-0
Molecular Formula:
C86H103Cl3N10O34P2
Molecular Weight:
1989.0911
IUPAC Name:
(1S,2R,18R,19R,22S,25R,28R,40S)-2-[(2R,4S,5R,6S)-4-amino-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-22-(2-amino-2-oxoethyl)-5,15-dichloro-48-[(2S,3R,4S,5S,6R)-3-[(2S,4S,5R,6S)-4-[[4-(4-chlorophenyl)phenyl]methylamino]-5-hydroxy-4,6-dimethyloxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-18,32,35,37-tetrahydroxy-19-[[(2R)-4-methyl-2-(methylamino)pentanoyl]amino]-20,23,26,42,44-pentaoxo-7,13-dioxa-21,24,27,41,43-pentazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta-3,5,8,10,12(48),14,16,29(45),30,32,34(39),35,37,46,49-pentadecaene-40-carboxylic acid;phosphoric acid
InChI:
InChI=1S/C86H97Cl3N10O26.2H3O4P/c1-35(2)22-51(92-7)77(110)98-67-69(105)42-15-20-55(49(88)24-42)120-57-26-44-27-58(73(57)125-84-74(71(107)70(106)59(34-100)122-84)124-62-32-86(6,76(109)37(4)119-62)93-33-38-8-10-39(11-9-38)40-12-17-45(87)18-13-40)121-56-21-16-43(25-50(56)89)72(123-61-31-85(5,91)75(108)36(3)118-61)68-82(115)97-66(83(116)117)48-28-46(101)29-54(103)63(48)47-23-41(14-19-53(47)102)64(79(112)99-68)96-80(113)65(44)95-78(111)52(30-60(90)104)94-81(67)114;2*1-5(2,3)4/h8-21,23-29,35-37,51-52,59,61-62,64-72,74-76,84,92-93,100-103,105-109H,22,30-34,91H2,1-7H3,(H2,90,104)(H,94,114)(H,95,111)(H,96,113)(H,97,115)(H,98,110)(H,99,112)(H,116,117);2*(H3,1,2,3,4)/t36-,37-,51+,52-,59+,61-,62-,64+,65+,66-,67+,68-,69+,70+,71-,72+,74+,75-,76-,84-,85-,86-;;/m0../s1
InChI Key:
PWTROOMOPLCZHB-BHYQHFGMSA-N
SMILES:
OP(=O)(O)O.OP(=O)(O)O.OC[C@H]1O[C@@H](Oc2c3Oc4ccc(cc4Cl)[C@@H](O)[C@@H](NC(=O)[C@@H](CC(C)C)NC)C(=O)N[C@H](C(=O)N[C@@H]4c(c3)cc2Oc2ccc(cc2Cl)[C@@H](O[C@@H]2O[C@@H](C)[C@@H]([C@@](C2)(C)N)O)[C@@H]2NC(=O)[C@H](NC4=O)c3ccc(c(c3)c3c(O)cc(cc3[C@H](NC2=O)C(=O)O)O)O)CC(=O)N)[C@@H]([C@H]([C@@H]1O)O)O[C@@H]1O[C@@H](C)[C@@H]([C@@](C1)(C)NCc1ccc(cc1)c1ccc(cc1)Cl)O
UNII:
VL1P93MKZN
Properties
Complexity:
3750
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
3
Defined Atom Stereocenter Count:
22
Defined Bond Stereocenter Count:
0
Exact Mass:
1986.518g/mol
Formal Charge:
0
Heavy Atom Count:
135
Hydrogen Bond Acceptor Count:
37
Hydrogen Bond Donor Count:
26
Isotope Atom Count:
0
Molecular Weight:
1989.104g/mol
Monoisotopic Mass:
1986.518g/mol
Rotatable Bond Count:
19
Topological Polar Surface Area:
717A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Effectiveness of a short (4 day) course of oritavancin in the treatment of simulated Clostridium difficile infection using a human gut model. | The Journal of antimicrobial chemotherapy 20121001 |
| Oritavancin: mechanism of action. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20120401 |
| In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20120401 |
| Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20120401 |
| Activity of oritavancin and comparators in vitro against standard and high inocula of Staphylococcus aureus. | International journal of antimicrobial agents 20120201 |
| In vitro time-kill analysis of oritavancin against clinical isolates of methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin. | Diagnostic microbiology and infectious disease 20111201 |
| MRSA new treatments on the horizon: current status. | Injury 20111201 |
| Oritavancin: a novel lipoglycopeptide active against Gram-positive pathogens including multiresistant strains. | International journal of antimicrobial agents 20101101 |
| Longitudinal analysis of the in vitro activity profile of oritavancin and comparator glycopeptides against Gram-positive organisms from Europe: 2005-2008. | International journal of antimicrobial agents 20101101 |
| Interactions of oritavancin, a new semi-synthetic lipoglycopeptide, with lipids extracted from Staphylococcus aureus. | Biochimica et biophysica acta 20101001 |
| New antibiotics for selective treatment of gastrointestinal infection caused by Clostridium difficile. | Expert opinion on therapeutic patents 20101001 |
| Visual compatibility of oritavancin diphosphate with selected coadministered drugs during simulated Y-site administration. | American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 20101001 |
| Oritavancin, a new lipoglycopeptide antibiotic: results from a thorough QT study. | Journal of clinical pharmacology 20100801 |
| In vitro activity of oritavancin against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA), vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA) and daptomycin-non-susceptible S. aureus (DNSSA). | International journal of antimicrobial agents 20100701 |
| New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. | Drugs 20100507 |
| Synthesis and in vitro evaluation of bisphosphonated glycopeptide prodrugs for the treatment of osteomyelitis. | Bioorganic & medicinal chemistry letters 20100215 |
| A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin. | Pharmacotherapy 20100101 |
| Comparative activity of oritavancin against meticillin-resistant Staphylococcus aureus (MRSA) bloodstream isolates from Geneva University Hospital. | International journal of antimicrobial agents 20091201 |
| Time-kill kinetics of oritavancin and comparator agents against Streptococcus pyogenes. | International journal of antimicrobial agents 20091201 |
| New antimicrobial agents for methicillin-resistant Staphylococcus aureus. | Critical care and resuscitation : journal of the Australasian Academy of Critical Care Medicine 20091201 |
| Vancomycin and oritavancin have different modes of action in Enterococcus faecium. | Journal of molecular biology 20091009 |
| Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. | The Journal of antimicrobial chemotherapy 20091001 |
| Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: Effect on membrane permeability and nanoscale lipid membrane organization. | Biochimica et biophysica acta 20090901 |
| Oritavancin binds to isolated protoplast membranes but not intact protoplasts of Staphylococcus aureus. | Journal of molecular biology 20090814 |
| Antimicrobial development in the era of emerging resistance. | Mini reviews in medicinal chemistry 20090701 |
| Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. | The Journal of antimicrobial chemotherapy 20090601 |
| New antibiotics for healthcare-associated pneumonia. | Seminars in respiratory and critical care medicine 20090201 |
| Regulatory watch: Non-inferiority-trial discussions impact new drug applications. | Nature reviews. Drug discovery 20090101 |
| Comparison of oritavancin versus vancomycin as treatments for clindamycin-induced Clostridium difficile PCR ribotype 027 infection in a human gut model. | The Journal of antimicrobial chemotherapy 20081101 |
| In vitro susceptibility of genotypically distinct and clonal Clostridium difficile strains to oritavancin. | The Journal of antimicrobial chemotherapy 20081001 |
| Oritavancin: a potential weapon in the battle against serious Gram-positive pathogens. | Future microbiology 20080601 |
| Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. | Journal of molecular biology 20080314 |
| Oritavancin: a new promising agent in the treatment of infections due to Gram-positive pathogens. | Expert opinion on investigational drugs 20080201 |
| Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. | Expert review of anti-infective therapy 20071201 |
| Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy. | Current opinion in investigational drugs (London, England : 2000) 20060801 |
| Conformational and quantitative characterization of oritavancin-peptidoglycan complexes in whole cells of Staphylococcus aureus by in vivo 13C and 15N labeling. | Journal of molecular biology 20060407 |
| Oritavancin--an investigational glycopeptide antibiotic. | Expert opinion on investigational drugs 20060401 |
| Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. | Antimicrobial agents and chemotherapy 20060301 |
| New antibiotics for the treatment of severe staphylococcal infection in the critically ill patient. | Current opinion in critical care 20051001 |
| Oritavancin: a new avenue for resistant Gram-positive bacteria. | Expert review of anti-infective therapy 20050601 |
| Pharmacokinetics of oritavancin in plasma and skin blister fluid following administration of a 200-milligram dose for 3 days or a single 800-milligram dose. | Antimicrobial agents and chemotherapy 20050101 |
| Recent advances in the treatment of infections due to resistant Staphylococcus aureus. | Current opinion in infectious diseases 20041201 |
| Glycopeptides in clinical development: pharmacological profile and clinical perspectives. | Current opinion in pharmacology 20041001 |
| Pharmacokinetics, safety, and tolerability of ascending single intravenous doses of oritavancin administered to healthy human subjects. | Diagnostic microbiology and infectious disease 20041001 |
| Current and new antimicrobial agents. | American heart journal 20040401 |
| Oritavancin and tigecycline: investigational antimicrobials for multidrug-resistant bacteria. | Pharmacotherapy 20040101 |
| Antibiotics as an adjunct to surgical management of lower extremity ulcerations. | Microsurgery 20040101 |
| Glycopeptide antibiotics: from conventional molecules to new derivatives. | Drugs 20040101 |
| Mechanism of action of oritavancin and related glycopeptide antibiotics. | FEMS microbiology reviews 20030101 |
| Effect of growth phase and pH on the in vitro activity of a new glycopeptide, oritavancin (LY333328), against Staphylococcus aureus and Enterococcus faecium. | The Journal of antimicrobial chemotherapy 20020701 |
| Vancomycin resistance: small molecule approaches targeting the bacterial cell wall biosynthesis. | Chembiochem : a European journal of chemical biology 20020402 |
| Glycopeptide derivatives. | Current medicinal chemistry 20011201 |
| Resistance to vancomycin, LY333328, ciprofloxacin and trovafloxacin of community-acquired and nosocomial strains of Enterococcus faecalis isolated in Badajoz (Spain) with and without high-level resistance to streptomycin and gentamicin. | Chemotherapy 20011201 |
| In vitro activity of LY333328 (oritavancin) against Gram-positive aerobic cocci and synergy with ciprofloxacin against enterococci. | The Journal of antimicrobial chemotherapy 20010801 |
| Oritavancin. Eli Lilly & Co. | Current opinion in investigational drugs (London, England : 2000) 20010801 |
| Effect of LY333328 against vancomycin-resistant Enterococcus faecium in a rat central venous catheter-associated infection model. | The Journal of antimicrobial chemotherapy 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.