200,000+ products from a single source!
sales@angenechem.com
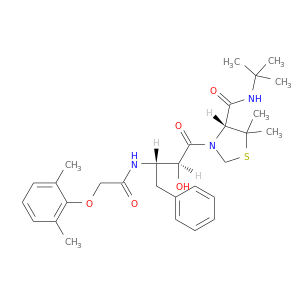
189357-33-3 | N-(3-((4R)-4-(N-(tert-Butyl)carbamoyl)-5,5-dimethyl(1,3-thiazolidin-3-yl))(1S,2S)-2-hydroxy-3-oxo-1-benzylpropyl)-2-(2,6-dimethylphenoxy)acetamide
CAS No: 189357-33-3 Catalog No: AG007T7G MDL No:
Product Description
Catalog Number:
AG007T7G
Chemical Name:
N-(3-((4R)-4-(N-(tert-Butyl)carbamoyl)-5,5-dimethyl(1,3-thiazolidin-3-yl))(1S,2S)-2-hydroxy-3-oxo-1-benzylpropyl)-2-(2,6-dimethylphenoxy)acetamide
CAS Number:
189357-33-3
Molecular Formula:
C30H41N3O5S
Molecular Weight:
555.7286
IUPAC Name:
(4R)-N-tert-butyl-3-[(2S,3S)-3-[[2-(2,6-dimethylphenoxy)acetyl]amino]-2-hydroxy-4-phenylbutanoyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxamide
InChI:
InChI=1S/C30H41N3O5S/c1-19-12-11-13-20(2)25(19)38-17-23(34)31-22(16-21-14-9-8-10-15-21)24(35)28(37)33-18-39-30(6,7)26(33)27(36)32-29(3,4)5/h8-15,22,24,26,35H,16-18H2,1-7H3,(H,31,34)(H,32,36)/t22-,24-,26+/m0/s1
InChI Key:
CSWRAOHDICNMPU-LLZJGCNPSA-N
SMILES:
O=C(N[C@H]([C@@H](C(=O)N1CSC([C@H]1C(=O)NC(C)(C)C)(C)C)O)Cc1ccccc1)COc1c(C)cccc1C
Properties
Complexity:
844
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
555.277g/mol
Formal Charge:
0
Heavy Atom Count:
39
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
555.734g/mol
Monoisotopic Mass:
555.277g/mol
Rotatable Bond Count:
10
Topological Polar Surface Area:
133A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.4
Literature
| Title | Journal |
|---|---|
| Small-sized human immunodeficiency virus type-1 protease inhibitors containing allophenylnorstatine to explore the S2' pocket. | Journal of medicinal chemistry 20091210 |
| Development of water-soluble prodrugs of the HIV-1 protease inhibitor KNI-727: importance of the conversion time for higher gastrointestinal absorption of prodrugs based on spontaneous chemical cleavage. | Journal of medicinal chemistry 20030911 |
| Identification and characterization of allophenylnorstatine-based inhibitors of plasmepsin II, an antimalarial target. | Biochemistry 20020219 |
| Design, synthesis, and biological evaluation of anti-HIV double-drugs. conjugates of HIV protease inhibitors with a reverse transcriptase inhibitor through spontaneously cleavable linkers. | Bioorganic & medicinal chemistry 20010601 |
| 'Double-Drugs'--a new class of prodrug form of an HIV protease inhibitor conjugated with a reverse transcriptase inhibitor by a spontaneously cleavable linker. | Bioorganic & medicinal chemistry letters 20000605 |
| Structure-activity relationship of small-sized HIV protease inhibitors containing allophenylnorstatine. | Journal of medicinal chemistry 19990520 |
| Small dipeptide-based HIV protease inhibitors containing the hydroxymethylcarbonyl isostere as an ideal transition-state mimic. | Biopolymers 19990101 |
| KNI-577, a potent small-sized HIV protease inhibitor based on the dipeptide containing the hydroxymethylcarbonyl isostere as an ideal transition-state mimic. | Archiv der Pharmazie 19980301 |
| Evaluation of anti-SIV potential of anti-neoplastic anthracyclines. | Proceedings of the Western Pharmacology Society 19920101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







