200,000+ products from a single source!
sales@angenechem.com
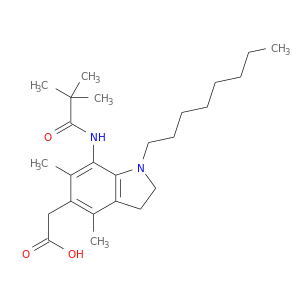
189198-30-9 | 1H-Indole-5-acetic acid, 7-[(2,2-dimethyl-1-oxopropyl)amino]-2,3-dihydro-4,6-dimethyl-1-octyl-
CAS No: 189198-30-9 Catalog No: AG002I1A MDL No:
Product Description
Catalog Number:
AG002I1A
Chemical Name:
1H-Indole-5-acetic acid, 7-[(2,2-dimethyl-1-oxopropyl)amino]-2,3-dihydro-4,6-dimethyl-1-octyl-
CAS Number:
189198-30-9
Molecular Formula:
C25H40N2O3
Molecular Weight:
416.5967
IUPAC Name:
2-[7-(2,2-dimethylpropanoylamino)-4,6-dimethyl-1-octyl-2,3-dihydroindol-5-yl]acetic acid
InChI:
InChI=1S/C25H40N2O3/c1-7-8-9-10-11-12-14-27-15-13-19-17(2)20(16-21(28)29)18(3)22(23(19)27)26-24(30)25(4,5)6/h7-16H2,1-6H3,(H,26,30)(H,28,29)
InChI Key:
TXIIZHHIOHVWJD-UHFFFAOYSA-N
SMILES:
CCCCCCCCN1CCc2c1c(NC(=O)C(C)(C)C)c(c(c2C)CC(=O)O)C
UNII:
D874R9PZ9T
Properties
Complexity:
571
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
416.304g/mol
Formal Charge:
0
Heavy Atom Count:
30
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
416.606g/mol
Monoisotopic Mass:
416.304g/mol
Rotatable Bond Count:
11
Topological Polar Surface Area:
69.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
6.3
Literature
| Title | Journal |
|---|---|
| Identification of unique expression signatures and therapeutic targets in esophageal squamous cell carcinoma. | BMC research notes 20120101 |
| Study on the hypochlolesterolemic and antioxidative effects of tyramine derivatives from the root bark of Lycium chenese Miller. | Nutrition research and practice 20111001 |
| A selective ACAT-1 inhibitor, K-604, stimulates collagen production in cultured smooth muscle cells and alters plaque phenotype in apolipoprotein E-knockout mice. | Atherosclerosis 20101101 |
| Novel acyl-CoA: cholesterol acyltransferase inhibitor: indoline-based sulfamide derivatives with low lipophilicity and protein binding ratio. | Chemical & pharmaceutical bulletin 20100801 |
| Novel tetrahydroisoquinoline derivatives with inhibitory activities against acyl-CoA: cholesterol acyltransferase and lipid peroxidation. | Chemical & pharmaceutical bulletin 20100801 |
| Membrane plasmalogen composition and cellular cholesterol regulation: a structure activity study. | Lipids in health and disease 20100101 |
| Impact of medical therapy on atheroma volume measured by different cardiovascular imaging modalities. | Cardiology research and practice 20100101 |
| Novel indoline-based acyl-CoA: cholesterol acyltransferase inhibitor: Effects of introducing a methanesulfonamide group on physicochemical properties and biological activities. | Bioorganic & medicinal chemistry 20090815 |
| Carotid atherosclerosis progression and ACAT inhibition. | JAMA 20090715 |
| Atherosclerosis drug fails to meet Phase III trial end point. | Nature reviews. Drug discovery 20090501 |
| ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: the CAPTIVATE randomized trial. | JAMA 20090318 |
| Substrate specificity, inhibitors and regulation of human cytochrome P450 2D6 and implications in drug development. | Current medicinal chemistry 20090101 |
| Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. | Diabetes 20081101 |
| Effect of CYP2D6 polymorphism on pharmacokinetics of a novel ACAT inhibitor, pactimibe and its unique metabolite, R-125528. | International journal of clinical pharmacology and therapeutics 20081101 |
| Novel binding mode of the acidic CYP2D6 substrates pactimibe and its metabolite R-125528. | Drug metabolism and disposition: the biological fate of chemicals 20080901 |
| Novel indoline-based acyl-CoA:cholesterol acyltransferase inhibitor with antiperoxidative activity: improvement of physicochemical properties and biological activities by introduction of carboxylic acid. | Journal of medicinal chemistry 20080814 |
| Effects of ketoconazole and quinidine on pharmacokinetics of pactimibe and its plasma metabolite, R-125528, in humans. | Drug metabolism and disposition: the biological fate of chemicals 20080801 |
| CYP2D6-Mediated metabolism of a novel acyl coenzyme A:cholesterol acyltransferase inhibitor, pactimibe, and its unique plasma metabolite, R-125528. | Drug metabolism and disposition: the biological fate of chemicals 20080301 |
| ACAT inhibitor pactimibe sulfate (CS-505) reduces and stabilizes atherosclerotic lesions by cholesterol-lowering and direct effects in apolipoprotein E-deficient mice. | Atherosclerosis 20070201 |
| [New agents against atherosclerosis tested. Alarming findings, ACAT inhibitors seem to have proatherogenic effects]. | Lakartidningen 20061001 |
| Multiple mechanisms of hypocholesterolemic action of pactimibe, a novel acyl-coenzyme A:cholesterol acyltransferase inhibitor. | European journal of pharmacology 20060814 |
| Importance of acyl-coenzyme A:cholesterol acyltransferase 1/2 dual inhibition for anti-atherosclerotic potency of pactimibe. | European journal of pharmacology 20060701 |
| Intravascular ultrasound assessment of novel antiatherosclerotic therapies: rationale and design of the Acyl-CoA:Cholesterol Acyltransferase Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Study. | American heart journal 20060701 |
| ACAT inhibition and the progression of coronary atherosclerosis. | The New England journal of medicine 20060615 |
| Pactimibe stabilizes atherosclerotic plaque through macrophage acyl-CoA:cholesterol acyltransferase inhibition in WHHL rabbits. | European journal of pharmacology 20060606 |
| Effect of ACAT inhibition on the progression of coronary atherosclerosis. | The New England journal of medicine 20060323 |
| Failure of ACAT inhibition to retard atherosclerosis. | The New England journal of medicine 20060323 |
| A bumpy road to breakthroughs. The news: it's hard to beat today's cardiac treatments. | Heart advisor 20060201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







