200,000+ products from a single source!
sales@angenechem.com
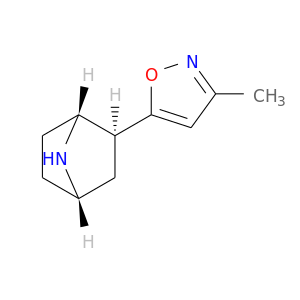
188895-96-7 | 7-Azabicyclo[2.2.1]heptane, 2-(3-methyl-5-isoxazolyl)-, (1R,2S,4S)-rel-
CAS No: 188895-96-7 Catalog No: AG002HGW MDL No:
Product Description
Catalog Number:
AG002HGW
Chemical Name:
7-Azabicyclo[2.2.1]heptane, 2-(3-methyl-5-isoxazolyl)-, (1R,2S,4S)-rel-
CAS Number:
188895-96-7
Molecular Formula:
C10H14N2O
Molecular Weight:
178.2310
IUPAC Name:
5-[(1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl]-3-methyl-1,2-oxazole
InChI:
InChI=1S/C10H14N2O/c1-6-4-10(13-12-6)8-5-7-2-3-9(8)11-7/h4,7-9,11H,2-3,5H2,1H3/t7-,8-,9+/m0/s1
InChI Key:
GEEFPQBPVBFCSD-XHNCKOQMSA-N
SMILES:
Cc1cc(on1)[C@H]1C[C@H]2N[C@@H]1CC2
Properties
Complexity:
209
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
178.111g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
178.235g/mol
Monoisotopic Mass:
178.111g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
38.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| The enantiomers of epiboxidine and of two related analogs: synthesis and estimation of their binding affinity at α4β2 and α7 neuronal nicotinic acetylcholine receptors. | Chirality 20120701 |
| Synthesis and binding affinity at α4β2 and α7 nicotinic acetylcholine receptors of new analogs of epibatidine and epiboxidine containing the 7-azabicyclo[2.2.1]hept-2-ene ring system. | Bioorganic & medicinal chemistry letters 20120115 |
| Determination of acid dissociation constants of compounds active at neuronal nicotinic acetylcholine receptors by means of electrophoretic and potentiometric techniques. | Analytical sciences : the international journal of the Japan Society for Analytical Chemistry 20100101 |
| New analogues of epiboxidine incorporating the 4,5-dihydroisoxazole nucleus: synthesis, binding affinity at neuronal nicotinic acetylcholine receptors, and molecular modeling investigations. | Chemistry & biodiversity 20090201 |
| Epiboxidine and novel-related analogues: a convenient synthetic approach and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes. | Bioorganic & medicinal chemistry letters 20080815 |
| Aza-Prins-pinacol approach to 7-azabicyclo[2.2.1]heptanes: syntheses of (+/-)-epibatidine and (+/-)-epiboxidine. | The Journal of organic chemistry 20071012 |
| Nicotine exposure refines visual map topography through an NMDA receptor-mediated pathway. | The European journal of neuroscience 20061201 |
| Synthesis and nicotinic acetylcholine receptor binding affinities of 2- and 3-isoxazolyl-8-azabicyclo[3.2.1]octanes. | Bioorganic & medicinal chemistry letters 20040405 |
| Homoepiboxidines: further potent agonists for nicotinic receptors. | Bioorganic & medicinal chemistry 20040102 |
| GABAergic systems modulate nicotinic receptor-mediated seizures in mice. | The Journal of pharmacology and experimental therapeutics 20030901 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







