200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 18771-50-1
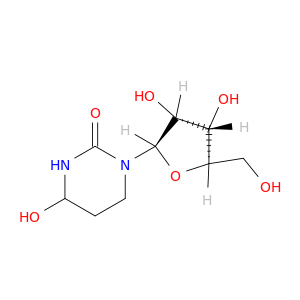
18771-50-1 | Tetrahydrouridine
CAS No: 18771-50-1 Catalog No: AG0038WM MDL No:
Product Description
Catalog Number:
AG0038WM
Chemical Name:
Tetrahydrouridine
CAS Number:
18771-50-1
Molecular Formula:
C9H16N2O6
Molecular Weight:
248.2331
IUPAC Name:
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one
InChI:
InChI=1S/C9H16N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h4-8,12-15H,1-3H2,(H,10,16)/t4-,5?,6-,7-,8-/m1/s1
InChI Key:
UCKYOOZPSJFJIZ-XVKVHKPRSA-N
SMILES:
OC[C@H]1O[C@H]([C@@H]([C@@H]1O)O)N1CCC(NC1=O)O
Properties
Complexity:
301
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
248.101g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
248.235g/mol
Monoisotopic Mass:
248.101g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
123A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.4
Literature
| Title | Journal |
|---|---|
| Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo. | International journal of cancer 20120701 |
| Effects of tetrahydrouridine on pharmacokinetics and pharmacodynamics of oral decitabine. | Blood 20120202 |
| Tetrahydrouridine inhibits cell proliferation through cell cycle regulation regardless of cytidine deaminase expression levels. | PloS one 20120101 |
| Comparison of in vitro metabolic conversion of capecitabine to 5-FU in rats, mice, monkeys and humans--toxicological implications. | The Journal of toxicological sciences 20110801 |
| Plasma pharmacokinetics and oral bioavailability of the 3,4,5,6-tetrahydrouridine (THU) prodrug, triacetyl-THU (taTHU), in mice. | Cancer chemotherapy and pharmacology 20110201 |
| An APCI LC-MS/MS method for routine determination of capecitabine and its metabolites in human plasma. | Journal of mass spectrometry : JMS 20100601 |
| Stability of 5-fluoro-2'-deoxycytidine and tetrahydrouridine in combination. | AAPS PharmSciTech 20100301 |
| Is the resistance of gemcitabine for pancreatic cancer settled only by overexpression of deoxycytidine kinase? | Oncology reports 20100201 |
| Synthesis of deoxytetrahydrouridine. | The Journal of organic chemistry 20090306 |
| Plasma pharmacokinetics and oral bioavailability of 3,4,5,6-tetrahydrouridine, a cytidine deaminase inhibitor, in mice. | Cancer chemotherapy and pharmacology 20080801 |
| Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). | Cancer chemotherapy and pharmacology 20080701 |
| Optimized blood sampling with cytidine deaminase inhibitor for improved analysis of capecitabine metabolites. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20080701 |
| Modulation of gemcitabine (2',2'-difluoro-2'-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. | Clinical cancer research : an official journal of the American Association for Cancer Research 20080601 |
| Quantitative determination of the cytidine deaminase inhibitor tetrahydrouridine (THU) in mouse plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. | Rapid communications in mass spectrometry : RCM 20070101 |
| The 1.48 A resolution crystal structure of the homotetrameric cytidine deaminase from mouse. | Biochemistry 20060627 |
| Relatively small increases in the steady-state levels of nucleobase deamination products in DNA from human TK6 cells exposed to toxic levels of nitric oxide. | Chemical research in toxicology 20060101 |
| Structure of human cytidine deaminase bound to a potent inhibitor. | Journal of medicinal chemistry 20050210 |
| Molecular characterization of thymidine kinase from Ureaplasma urealyticum: nucleoside analogues as potent inhibitors of mycoplasma growth. | Molecular microbiology 20031101 |
| Epimer interconversion, isomerization, and hydrolysis of tetrahydrouridine: implications for cytidine deaminase inhibition. | Journal of pharmaceutical sciences 20031001 |
| Analytical and pharmacokinetic studies with 5-chloro-2'-deoxycytidine. | Biochemical pharmacology 20021115 |
| Five-chlorodeoxycytidine, a tumor-selective enzyme-driven radiosensitizer, effectively controls five advanced human tumors in nude mice. | International journal of radiation oncology, biology, physics 20011101 |
| Regulatory effects of deoxyribonucleosides on the activity of 5-methoxymethyl-2'-deoxycytidine: modulation of antiherpes activity by deoxyguanosine and tetrahydrodeoxyuridine. | Antiviral research 19910501 |
| Antiherpes virus activity and effect on deoxyribonucleoside triphosphate pools of (E)-5-(2-bromovinyl)-2'-deoxycytidine in combination with deaminase inhibitors. | Antiviral research 19900301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.