200,000+ products from a single source!
sales@angenechem.com
Home > Pyridines > 180384-56-9
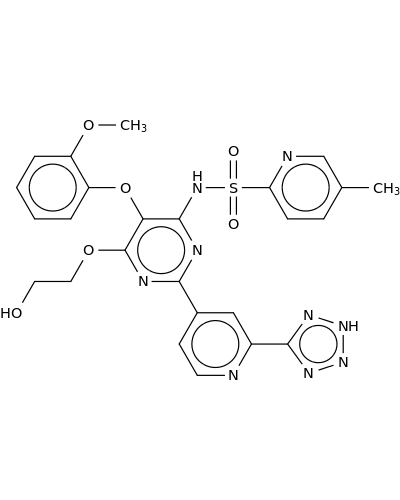
180384-56-9 | 2-Pyridinesulfonamide, N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)-4-pyridinyl]-4-pyrimidinyl]-5-methyl-
CAS No: 180384-56-9 Catalog No: AG0021Y5 MDL No:
Product Description
Catalog Number:
AG0021Y5
Chemical Name:
2-Pyridinesulfonamide, N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)-4-pyridinyl]-4-pyrimidinyl]-5-methyl-
CAS Number:
180384-56-9
Molecular Formula:
C25H23N9O6S
Molecular Weight:
577.5718
IUPAC Name:
N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)pyridin-4-yl]pyrimidin-4-yl]-5-methylpyridine-2-sulfonamide
InChI:
InChI=1S/C25H23N9O6S/c1-15-7-8-20(27-14-15)41(36,37)32-24-21(40-19-6-4-3-5-18(19)38-2)25(39-12-11-35)29-22(28-24)16-9-10-26-17(13-16)23-30-33-34-31-23/h3-10,13-14,35H,11-12H2,1-2H3,(H,28,29,32)(H,30,31,33,34)
InChI Key:
LFWCJABOXHSRGC-UHFFFAOYSA-N
SMILES:
OCCOc1nc(nc(c1Oc1ccccc1OC)NS(=O)(=O)c1ccc(cn1)C)c1ccnc(c1)c1nn[nH]n1
UNII:
3DRR0X4728
Properties
Complexity:
917
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
577.149g/mol
Formal Charge:
0
Heavy Atom Count:
41
Hydrogen Bond Acceptor Count:
14
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
577.576g/mol
Monoisotopic Mass:
577.149g/mol
Rotatable Bond Count:
11
Topological Polar Surface Area:
209A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.6
Literature
| Title | Journal |
|---|---|
| Global cerebral atrophy after subarachnoid hemorrhage: a possible marker of acute brain injury and assessment of its impact on outcome. | Acta neurochirurgica. Supplement 20130101 |
| Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). | Acta neurochirurgica. Supplement 20130101 |
| Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. | Stroke 20121001 |
| Endothelin receptor antagonists for subarachnoid hemorrhage. | The Cochrane database of systematic reviews 20120912 |
| Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. | British journal of anaesthesia 20120901 |
| Electrocardiographic changes predict angiographic vasospasm after aneurysmal subarachnoid hemorrhage. | Stroke 20120801 |
| Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. | Stroke 20120601 |
| Novel treatments for vasospasm after subarachnoid hemorrhage. | Current opinion in critical care 20120401 |
| Quality of life and healthcare resource use associated with angiographic vasospasm after aneurysmal subarachnoid hemorrhage. | Stroke 20120401 |
| From bench-to-bedside in catastrophic cerebrovascular disease: development of drugs targeting the endothelin axis in subarachnoid hemorrhage-related vasospasm. | Neurological research 20120301 |
| Method of aneurysm treatment does not affect clot clearance after aneurysmal subarachnoid hemorrhage. | Neurosurgery 20120101 |
| Attributing hypodensities on CT to angiographic vasospasm is not sensitive and unreliable. | Stroke 20120101 |
| Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. | Experimental neurology 20120101 |
| Intracranial drug delivery for subarachnoid hemorrhage. | Therapeutic delivery 20120101 |
| Effect of clazosentan in patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. | PloS one 20120101 |
| Interobserver variability in the interpretation of computed tomography following aneurysmal subarachnoid hemorrhage. | Journal of neurosurgery 20111201 |
| The effect of endothelin receptor antagonists on vasospasm following aneurysmal subarachnoid hemorrhage. | Neurosurgery 20111201 |
| Stroke: disappointing results for clazosentan in CONSCIOUS-2. | Nature reviews. Neurology 20111018 |
| Macitentan: entry-into-humans study with a new endothelin receptor antagonist. | European journal of clinical pharmacology 20111001 |
| Clazosentan for patients with subarachnoid haemorrhage: lessons learned. | The Lancet. Neurology 20111001 |
| Effect of clazosentan, a selective endothelin A receptor antagonist, and tezosentan, a dual endothelin A/B antagonist, on pulsatile shear stress induced constriction of the iliac in the anaesthetized pig. | Clinical and experimental pharmacology & physiology 20110801 |
| Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). | The Lancet. Neurology 20110701 |
| Different effects of clazosentan on consequences of subarachnoid hemorrhage in rats. | Brain research 20110525 |
| Dissociation of vasospasm and secondary effects of experimental subarachnoid hemorrhage by clazosentan. | Stroke 20110501 |
| Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. | Stroke 20110401 |
| [Protective measures against cerebral ischemia following subarachnoid hemorrhage: Part 1]. | Revista espanola de anestesiologia y reanimacion 20110401 |
| [Protective measures against cerebral ischemia following subarachnoid hemorrhage: Part 2]. | Revista espanola de anestesiologia y reanimacion 20110401 |
| Influence of severe renal impairment on the pharmacokinetics of clazosentan. | Journal of clinical pharmacology 20110301 |
| Clazosentan, a novel endothelin A antagonist, improves cerebral blood flow and behavior after traumatic brain injury. | Neurological research 20110301 |
| Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. | Molecular neurobiology 20110201 |
| Hypoperfusion in the acute phase of subarachnoid hemorrhage. | Acta neurochirurgica. Supplement 20110101 |
| Clazosentan: prevention of cerebral vasospasm and the potential to overcome infarction. | Acta neurochirurgica. Supplement 20110101 |
| Influence of different degrees of liver impairment on the pharmacokinetics of clazosentan. | British journal of clinical pharmacology 20110101 |
| Human cerebrovascular contractile receptors are upregulated via a B-Raf/MEK/ERK-sensitive signaling pathway. | BMC neuroscience 20110101 |
| Is vasospasm actually bad for you? | World neurosurgery 20110101 |
| Inhibition of cerebrovascular raf activation attenuates cerebral blood flow and prevents upregulation of contractile receptors after subarachnoid hemorrhage. | BMC neuroscience 20110101 |
| Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. | Neurocritical care 20101201 |
| Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. | Neurocritical care 20101001 |
| Patient age and vasospasm after subarachnoid hemorrhage. | Neurosurgery 20101001 |
| Effect of endothelin-receptor antagonists on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage remains unclear. | Stroke 20091201 |
| Therapeutic approaches to cerebral vasospasm complicating ruptured aneurysm. | Neurology international 20091116 |
| Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. | Brain : a journal of neurology 20090701 |
| In vitro and in vivo pharmacokinetic characteristics of clazosentan, an intravenous endothelin receptor antagonist, in humans. | International journal of clinical pharmacology and therapeutics 20090301 |
| Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: is angiographic vasospasm an epiphenomenon? | Stroke 20090201 |
| Diagnosis and management of vasospasm. | F1000 medicine reports 20090101 |
| Clazosentan, an endothelin receptor antagonist, prevents early hypoperfusion during the acute phase of massive experimental subarachnoid hemorrhage: a laser Doppler flowmetry study in rats. | Journal of neurosurgery 20081201 |
| Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. | Stroke 20081101 |
| Clazosentan: an endothelin receptor antagonist for treatment of vasospasm after subarachnoid hemorrhage. | Expert opinion on investigational drugs 20081101 |
| IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. | Proceedings of the National Academy of Sciences of the United States of America 20080219 |
| Vasospasm after aneurysmal subarachnoid hemorrhage: need for further study. | Acta neurochirurgica. Supplement 20080101 |
| Influence of ethnic origin and sex on the pharmacokinetics of clazosentan. | Journal of clinical pharmacology 20071101 |
| [Systemic treatments of the vasospasm]. | Annales francaises d'anesthesie et de reanimation 20071101 |
| Effect of delayed cerebral vasospasm on cerebrovascular endothelin A receptor expression and function. | Journal of neurosurgery 20070701 |
| Cerebral vasospasm: looking beyond vasoconstriction. | Trends in pharmacological sciences 20070601 |
| Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. | Nature clinical practice. Neurology 20070501 |
| Tolerability, pharmacokinetics, and pharmacodynamics of clazosentan, a parenteral endothelin receptor antagonist. | European journal of clinical pharmacology 20070201 |
| Effects of the selective endothelin A (ET(A)) receptor antagonist Clazosentan on cerebral perfusion and cerebral oxygenation following severe subarachnoid hemorrhage - preliminary results from a randomized clinical series. | Acta neurochirurgica 20070101 |
| Clinical review: Prevention and therapy of vasospasm in subarachnoid hemorrhage. | Critical care (London, England) 20070101 |
| Effect of gender on the tolerability, safety and pharmacokinetics of clazosentan following long-term infusion. | Clinical drug investigation 20070101 |
| Endothelin receptor antagonists. | Pharmacology & therapeutics 20060601 |
| Profile of past and current clinical trials involving endothelin receptor antagonists: the novel '-sentan' class of drug. | Experimental biology and medicine (Maywood, N.J.) 20060601 |
| Clazosentan (Actelion). | Current opinion in investigational drugs (London, England : 2000) 20060301 |
| Vasospasm. | Journal of neurosurgery 20050701 |
| Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. | Journal of neurosurgery 20050701 |
| Cerebrovascular characterization of clazosentan, the first nonpeptide endothelin receptor antagonist clinically effective for the treatment of cerebral vasospasm. Part I: inhibitory effect on endothelin(A) receptor-mediated contraction. | Journal of neurosurgery 20050601 |
| Cerebrovascular characterization of clazosentan, the first nonpeptide endothelin receptor antagonist shown to be clinically effective for the treatment of cerebral vasospasm. Part II: effect on endothelin(B) receptor-mediated relaxation. | Journal of neurosurgery 20050601 |
| Effects of endothelin-1 and endothelin-1-receptor blockade on renal function in humans. | Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 20041101 |
| Pathophysiological plasma ET-1 levels antagonize beta-adrenergic dilation of coronary resistance vessels in conscious dogs. | American journal of physiology. Heart and circulatory physiology 20041001 |
| Effects of endothelin-1 and endothelin-1 receptor blockade on cardiac output, aortic pressure, and pulse wave velocity in humans. | Hypertension (Dallas, Tex. : 1979) 20030601 |
| Effect of Ro 61-1790, a selective endothelin-A receptor antagonist, on systemic and uterine hemodynamics and fetal oxygenation in sheep. | American journal of obstetrics and gynecology 20020101 |
| Inhibition of endothelin-1 by the competitive ET(A) receptor antagonist Ro 61-1790 reduces lesion volume after cold injury in the rat. | Pflugers Archiv : European journal of physiology 20010301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.