200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 180-84-7
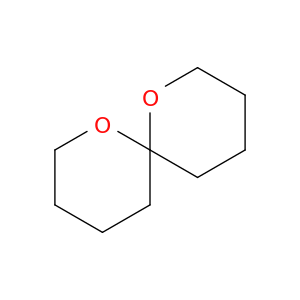
180-84-7 | 1,7-Dioxaspiro[5.5]Undecane
CAS No: 180-84-7 Catalog No: AG0021KI MDL No:
Product Description
Catalog Number:
AG0021KI
Chemical Name:
1,7-Dioxaspiro[5.5]Undecane
CAS Number:
180-84-7
Molecular Formula:
C9H16O2
Molecular Weight:
156.2221
IUPAC Name:
1,7-dioxaspiro[5.5]undecane
InChI:
InChI=1S/C9H16O2/c1-3-7-10-9(5-1)6-2-4-8-11-9/h1-8H2
InChI Key:
GBBVHDGKDQAEOT-UHFFFAOYSA-N
SMILES:
C1CCC2(OC1)CCCCO2
EC Number:
205-870-7
UNII:
KHI3QEQ4ZJ
Properties
Complexity:
118
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
156.115g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
156.225g/mol
Monoisotopic Mass:
156.115g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
18.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.6
Literature
| Title | Journal |
|---|---|
| Analyzing diurnal and age-related pheromone emission of the olive fruit fly, Bactrocera oleae by sequential SPME-GCMS analysis. | Journal of chemical ecology 20120801 |
| (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: first evidence of a male-produced female attractant in olive fruit fly. | Die Naturwissenschaften 20120101 |
| Efficacy of new mass-trapping devices against Bactrocera oleae (Diptera tephritidae) for minimizing pesticide input in agroecosystems. | Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes 20090601 |
| Energetics of oxaspirocycle prototypes: 1,7-dioxaspiro[5.5]undecane and 1,7,9-trioxadispiro[5.1.5.3]hexadecane. | The Journal of organic chemistry 20061124 |
| Spiroacetal biosynthesis: (+/-)-1,7-dioxaspiro[5.5]undecane in Bactrocera cacuminata and Bactrocera oleae (Olive Fruit Fly). | Organic letters 20050317 |
| Synthesis of haptens and development of an immunoassay for the olive fruit fly pheromone. | Journal of agricultural and food chemistry 20040714 |
| [(18)O]-oxygen incorporation reveals novel pathways in spiroacetal biosynthesis by Bactrocera cacuminata and B. cucumis. | Journal of the American Chemical Society 20020703 |
| Sex pheromone biosynthesis in the female olive fruit-fly. Double labelling from [18O2]-dioxygen into 1,7-dioxaspiro[5.5]undecane. | Chemical communications (Cambridge, England) 20020621 |
| Combinatorial synthesis of natural product-like molecules using a first-generation spiroketal scaffold. | Journal of combinatorial chemistry 20020101 |
| Non-covalent interactions in the crystallization of the enantiomers of 1,7-dioxaspiro. | Acta crystallographica. Section B, Structural science 20010601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.