200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 179420-17-8
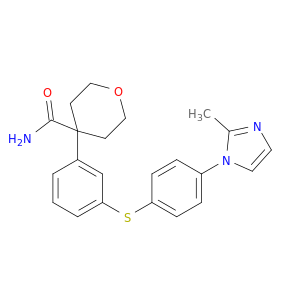
179420-17-8 | 2H-Pyran-4-carboxamide, tetrahydro-4-[3-[[4-(2-methyl-1H-imidazol-1-yl)phenyl]thio]phenyl]-
CAS No: 179420-17-8 Catalog No: AG002704 MDL No:
Product Description
Catalog Number:
AG002704
Chemical Name:
2H-Pyran-4-carboxamide, tetrahydro-4-[3-[[4-(2-methyl-1H-imidazol-1-yl)phenyl]thio]phenyl]-
CAS Number:
179420-17-8
Molecular Formula:
C22H23N3O2S
Molecular Weight:
393.5019
IUPAC Name:
4-[3-[4-(2-methylimidazol-1-yl)phenyl]sulfanylphenyl]oxane-4-carboxamide
InChI:
InChI=1S/C22H23N3O2S/c1-16-24-11-12-25(16)18-5-7-19(8-6-18)28-20-4-2-3-17(15-20)22(21(23)26)9-13-27-14-10-22/h2-8,11-12,15H,9-10,13-14H2,1H3,(H2,23,26)
InChI Key:
VPTONMHDLLMOOV-UHFFFAOYSA-N
SMILES:
Cc1nccn1c1ccc(cc1)Sc1cccc(c1)C1(CCOCC1)C(=O)N
UNII:
5275PJ1C59
Properties
Complexity:
532
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
393.151g/mol
Formal Charge:
0
Heavy Atom Count:
28
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
393.505g/mol
Monoisotopic Mass:
393.151g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
95.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.2
Literature
| Title | Journal |
|---|---|
| In vitro-in vivo correlation and translation to the clinical outcome for CJ-13,610, a novel inhibitor of 5-lipoxygenase. | Drug metabolism and disposition: the biological fate of chemicals 20100701 |
| Pharmacophore modeling and virtual screening for designing potential 5-lipoxygenase inhibitors. | Bioorganic & medicinal chemistry letters 20100201 |
| CJ-13610, an orally active inhibitor of 5-lipoxygenase is efficacious in preclinical models of pain. | European journal of pharmacology 20090901 |
| The use of gastrointestinal intubation studies for controlled release development. | British journal of clinical pharmacology 20090901 |
| A rat air pouch model for evaluating the efficacy and selectivity of 5-lipoxygenase inhibitors. | European journal of pharmacology 20080414 |
| Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor. | The Journal of pharmacology and experimental therapeutics 20071201 |
| Dog colonoscopy model for predicting human colon absorption. | Pharmaceutical research 20060701 |
| Optimization of imidazole 5-lipoxygenase inhibitors and selection and synthesis of a development candidate. | Chemical & pharmaceutical bulletin 20050801 |
| 5-Lipoxygenase inhibitors: convenient synthesis of 4-[3-(4-heterocyclylphenylthio)phenyl]-3,4,5,6-tetrahydro-2H-pyran-4-carboxamide analogues. | Bioorganic & medicinal chemistry letters 20050516 |
| Molecular pharmacological profile of the nonredox-type 5-lipoxygenase inhibitor CJ-13,610. | British journal of pharmacology 20040701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.