200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1792-81-0
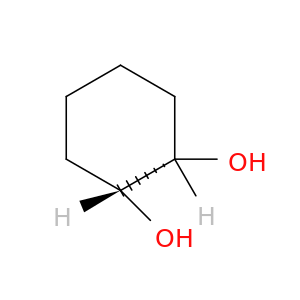
1792-81-0 | 1,2-Cyclohexanediol, (1R,2S)-rel-
CAS No: 1792-81-0 Catalog No: AG0026V5 MDL No:MFCD00064944
Product Description
Catalog Number:
AG0026V5
Chemical Name:
1,2-Cyclohexanediol, (1R,2S)-rel-
CAS Number:
1792-81-0
Molecular Formula:
C6H12O2
Molecular Weight:
116.1583
MDL Number:
MFCD00064944
IUPAC Name:
(1R,2S)-cyclohexane-1,2-diol
InChI:
InChI=1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6+
InChI Key:
PFURGBBHAOXLIO-OLQVQODUSA-N
SMILES:
O[C@@H]1CCCC[C@@H]1O
Properties
Complexity:
62.9
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
116.084g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
116.16g/mol
Monoisotopic Mass:
116.084g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
40.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.2
Literature
| Title | Journal |
|---|---|
| Assessment of the nuclear pore dilating agent trans-cyclohexane-1,2-diol in differentiated airway epithelium. | The journal of gene medicine 20120701 |
| Theoretical study of oxidation of cyclohexane diol to adipic anhydride by [Ru(IV)(O)(tpa)(H2O)]2+ complex (tpa ═ tris(2-pyridylmethyl)amine). | Inorganic chemistry 20110704 |
| A very active cu-catalytic system for the synthesis of aryl, heteroaryl, and vinyl sulfides. | The Journal of organic chemistry 20100604 |
| On the origins of kinetic resolution of cyclohexane-1,2-diols through stereoselective acylation by chiral tetrapeptides. | Organic letters 20090806 |
| From simple diols to carbohydrate derivatives of phenylarsonic acid. | Inorganic chemistry 20090202 |
| Oxidation of cyclohexanediol derivatives with 12-tungstophosphoric acid-hydrogen peroxide system. | Journal of oleo science 20090101 |
| Advantages of synthesizing trans-1,2-cyclohexanediol in a continuous flow microreactor over a standard glass apparatus. | The Journal of organic chemistry 20071221 |
| Biocatalytic production of enantiopure cyclohexane-trans-1,2-diol using extracellular lipases from Bacillus subtilis. | Applied microbiology and biotechnology 20061001 |
| Absence of interactive effects of trans-1,2-cyclohexanediol, a major metabolite of the side-chain of candesartan cilexetil, on digoxin-induced arrhythmias in dogs. | Journal of pharmacological sciences 20030801 |
| Tin(II) chloride catalyzed reactions of diazodiphenylmethane with vicinal diols in an aprotic solvent. The reactions with cis- and trans-1,2-cyclohexanediols and 1,2-propanediol. | Carbohydrate research 20030422 |
| Expression of benzene dioxygenase from Pseudomonas putida ML2 in cis-1,2-cyclohexanediol-degrading pseudomonads. | Applied microbiology and biotechnology 20010601 |
| Asymmetric synthesis of alpha,alpha-disubstituted alpha-amino acids using (S,S)-cyclohexane-1,2-diol as a chiral auxiliary. | The Journal of organic chemistry 20010420 |
Related Products
© 2019 Angene International Limited. All rights Reserved.