200,000+ products from a single source!
sales@angenechem.com
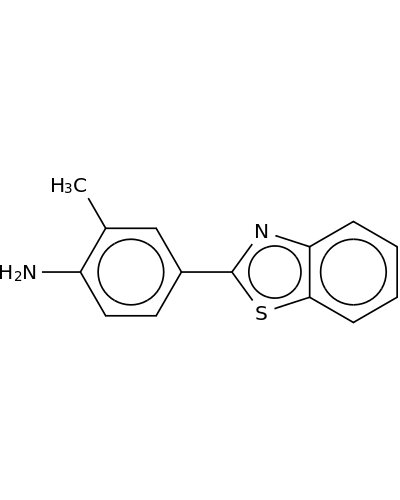
178804-04-1 | Benzenamine, 4-(2-benzothiazolyl)-2-methyl-
CAS No: 178804-04-1 Catalog No: AG0026BR MDL No:MFCD00950805
Product Description
Catalog Number:
AG0026BR
Chemical Name:
Benzenamine, 4-(2-benzothiazolyl)-2-methyl-
CAS Number:
178804-04-1
Molecular Formula:
C14H12N2S
Molecular Weight:
240.3235
MDL Number:
MFCD00950805
IUPAC Name:
4-(1,3-benzothiazol-2-yl)-2-methylaniline
InChI:
InChI=1S/C14H12N2S/c1-9-8-10(6-7-11(9)15)14-16-12-4-2-3-5-13(12)17-14/h2-8H,15H2,1H3
InChI Key:
IDBCUMFOZBUJCL-UHFFFAOYSA-N
SMILES:
Nc1ccc(cc1C)c1nc2c(s1)cccc2
NSC Number:
674495
Properties
Complexity:
271
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
240.072g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
240.324g/mol
Monoisotopic Mass:
240.072g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
67.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| Nanoliposomes for encapsulation and delivery of the potential antitumoral methyl 6-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carboxylate. | Nanoscale research letters 20110101 |
| The role of aryl hydrocarbon receptor and crosstalk with estrogen receptor in response of breast cancer cells to the novel antitumor agents benzothiazoles and aminoflavone. | International journal of breast cancer 20110101 |
| Synthesis, and biological evaluation of 2-(4-aminophenyl)benzothiazole derivatives as photosensitizing agents. | Bioorganic & medicinal chemistry 20100815 |
| Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. | BMC cancer 20090101 |
| BRCA1 transcriptional activity is enhanced by interactions between its AD1 domain and AhR. | Cancer chemotherapy and pharmacology 20081101 |
| Mechanisms of acquired resistance to 2-(4-Amino-3-methylphenyl)benzothiazole in breast cancer cell lines. | Breast cancer research and treatment 20080701 |
| Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. | Breast cancer research : BCR 20080101 |
| BRCA1 modulates xenobiotic stress-inducible gene expression by interacting with ARNT in human breast cancer cells. | The Journal of biological chemistry 20060526 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. | Journal of medicinal chemistry 20060112 |
| Fluorinated 2-(4-amino-3-methylphenyl)benzothiazoles induce CYP1A1 expression, become metabolized, and bind to macromolecules in sensitive human cancer cells. | Drug metabolism and disposition: the biological fate of chemicals 20041201 |
| The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. | Current medicinal chemistry 20040401 |
| Synthesis and SAR of 2-arylbenzoxazoles, benzothiazoles and benzimidazoles as inhibitors of lysophosphatidic acid acyltransferase-beta. | Bioorganic & medicinal chemistry letters 20040322 |
| Professor Tom Connors and the development of novel cancer therapies by the Phase I/II Clinical Trials Committee of Cancer Research UK. | British journal of cancer 20030804 |
| Halothane, a novel solvent for the preparation of liposomes containing 2-4'-amino-3'-methylphenyl benzothiazole (AMPB), an anticancer drug: a technical note. | AAPS PharmSciTech 20030601 |
| DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. | British journal of cancer 20030224 |
| Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. | British journal of cancer 20030210 |
| In vitro evaluation of amino acid prodrugs of novel antitumour 2-(4-amino-3-methylphenyl)benzothiazoles. | British journal of cancer 20020422 |
| Preclinical evaluation of amino acid prodrugs of novel antitumor 2-(4-amino-3-methylphenyl)benzothiazoles. | Molecular cancer therapeutics 20020201 |
| Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. | Journal of medicinal chemistry 20020131 |
| Aryl hydrocarbon receptor mediates sensitivity of MCF-7 breast cancer cells to antitumor agent 2-(4-amino-3-methylphenyl) benzothiazole. | Molecular pharmacology 20020101 |
| Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. | Journal of medicinal chemistry 20010426 |
| Antitumour benzothiazoles. Part 15: The synthesis and physico-chemical properties of 2-(4-aminophenyl)benzothiazole sulfamate salt derivatives. | Bioorganic & medicinal chemistry letters 20010423 |
| The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) and related compounds. | Current medicinal chemistry 20010201 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.