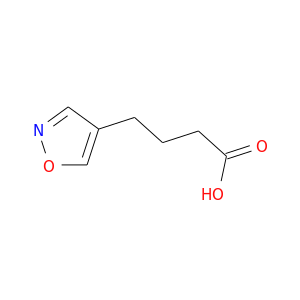
200,000+ products from a single source!
sales@angenechem.com
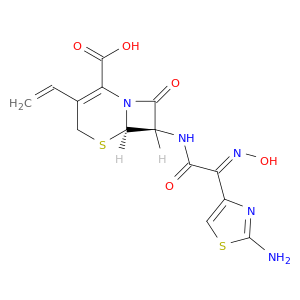
178601-88-2 | 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2E)-2-(2-amino-4-thiazolyl)-2-(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-, (6R,7R)-
CAS No: 178601-88-2 Catalog No: AG002661 MDL No:
Product Description
Catalog Number:
AG002661
Chemical Name:
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2E)-2-(2-amino-4-thiazolyl)-2-(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-, (6R,7R)-
CAS Number:
178601-88-2
Molecular Formula:
C14H13N5O5S2
Molecular Weight:
395.4135
IUPAC Name:
(6R,7R)-7-[[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-hydroxyiminoacetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
InChI:
InChI=1S/C14H13N5O5S2/c1-2-5-3-25-12-8(11(21)19(12)9(5)13(22)23)17-10(20)7(18-24)6-4-26-14(15)16-6/h2,4,8,12,24H,1,3H2,(H2,15,16)(H,17,20)(H,22,23)/b18-7+/t8-,12-/m1/s1
InChI Key:
RTXOFQZKPXMALH-RWFJUVPESA-N
SMILES:
O/N=C(\c1csc(n1)N)/C(=O)N[C@@H]1C(=O)N2[C@@H]1SCC(=C2C(=O)O)C=C
UNII:
61G2M33IGF
Properties
Complexity:
739
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
1
Exact Mass:
395.036g/mol
Formal Charge:
0
Heavy Atom Count:
26
Hydrogen Bond Acceptor Count:
10
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
395.408g/mol
Monoisotopic Mass:
395.036g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
212A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0
Literature
| Title | Journal |
|---|---|
| Comparison of amoxicillin/clavulanic acid high dose with cefdinir in the treatment of acute otitis media. | Drugs 20121022 |
| Development and validation of a rapid HPLC method for the determination of cefdinir in beagle dog plasma integrated with an automatic on-line solid-phase extraction following protein precipitation in the 96-well plate format. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20120501 |
| [Identification of impurity peaks in the HPLC chromatogram by LC-MS and two-dimensional chromatographic correlation spectroscopy]. | Yao xue xue bao = Acta pharmaceutica Sinica 20120401 |
| Bioequivalence evaluation of cefdinir in healthy fasting subjects. | Arzneimittel-Forschung 20120101 |
| Simultaneous determination of cefdinir and cefixime in human plasma by RP-HPLC/UV detection method: Method development, optimization, validation, and its application to a pharmacokinetic study. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20110815 |
| Oral antibiotics for infections due to multidrug-resistant Gram-negative organisms. | Scandinavian journal of infectious diseases 20110801 |
| Prophylactic cefdinir for pediatric cases of complicated urinary tract infection. | Pediatrics international : official journal of the Japan Pediatric Society 20110201 |
| Preparation and pharmaceutical characterization of amorphous cefdinir using spray-drying and SAS-process. | International journal of pharmaceutics 20100830 |
| Another vacation, another medical 'crisis'. | Nature reviews. Gastroenterology & hepatology 20100401 |
| In vitro antimicrobial activity of cefditoren and other oral antibiotics against Streptococcus pneumoniae, isolated from children with community acquired respiratory tract infections. | The Japanese journal of antibiotics 20100201 |
| Infant male with blood-colored stools. | Annals of emergency medicine 20090801 |
| Rate of eradication of group A beta-hemolytic streptococci in children with pharyngo-tonsillitis by amoxicillin and cefdinir. | International journal of pediatric otorhinolaryngology 20090501 |
| Visual diagnosis: four infants who have red, 'bloody' stools. | Pediatrics in review 20090401 |
| Cefdinir-induced hepatotoxicity: potential hazards of inappropriate antibiotic use. | Journal of general internal medicine 20081101 |
| Cidal activity of oral third-generation cephalosporins against Streptococcus pneumoniae in relation to cefotaxime intrinsic activity. | European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 20080801 |
| Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. | Journal of pharmaceutical and biomedical analysis 20080715 |
| Nonbloody, red stools from coadministration of cefdinir and iron-supplemented infant formulas. | Pharmacotherapy 20080501 |
| A favorable response to steroid therapy in a child with drug-associated acute vanishing bile duct syndrome and skin disorder. | Journal of paediatrics and child health 20080401 |
| Voltammetric behavior of cefdinir in solubilized system. | Journal of colloid and interface science 20080215 |
| Cefdinir-associated 'bloody stools' in an infant. | Journal of the American Board of Family Medicine : JABFM 20080101 |
| Comparative bioavailability of two cefdinir suspension formulations in Middle Eastern healthy volunteers after single oral administration. | Arzneimittel-Forschung 20080101 |
| Effect of L-phenylalanine supplementation and a high-protein diet on pharmacokinetics of cefdinir in healthy volunteers: an exploratory study. | Journal of clinical pharmacy and therapeutics 20070601 |
| Isolation, structural elucidation and characterization of impurities in Cefdinir. | Journal of pharmaceutical and biomedical analysis 20070312 |
| Cefdinir: an oral cephalosporin for the treatment of respiratory tract infections and skin and skin structure infections. | Expert review of anti-infective therapy 20070201 |
| Bayesian estimation of intervention effect with pre- and post-misclassified binomial data. | Journal of biopharmaceutical statistics 20070101 |
| Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. | Current medical research and opinion 20061201 |
| Therapeutic experience with cefdinir in the treatment of uSSSIs. | International journal of clinical practice 20061001 |
| Cefdinir: A comparative study of anhydrous vs. monohydrate form. Microstructure and tabletting behaviour. | European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 20061001 |
| Efficacy, tolerability, and parent reported outcomes for cefdinir vs. high-dose amoxicillin/clavulanate oral suspension for acute otitis media in young children. | Current medical research and opinion 20060901 |
| Update on the cefdinir spectrum and potency against pathogens isolated from uncomplicated skin and soft tissue infections in North America: are we evaluating the orally administered cephalosporins correctly? | Diagnostic microbiology and infectious disease 20060801 |
| The efficacy of cefdinir in acute bacterial rhinosinusitis. | Expert opinion on pharmacotherapy 20060601 |
| Selective method for the determination of cefdinir in human plasma using liquid chromatography electrospray ionization tandam mass spectrometry. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20060413 |
| Cefdinir pharmacokinetics and tolerability in children receiving 25 mg/kg once daily. | The Pediatric infectious disease journal 20060301 |
| A multicenter, open label, double tympanocentesis study of high dose cefdinir in children with acute otitis media at high risk of persistent or recurrent infection. | The Pediatric infectious disease journal 20060301 |
| Susceptibility of recent bacterial isolates to cefdinir and selected antibiotics among children with urinary tract infections. | Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 20060101 |
| Microdialysis studies of the distribution of antibiotics into chinchilla middle ear fluid. | Pharmacotherapy 20051201 |
| A pooled analysis of seven randomized crossover studies of the palatability of cefdinir oral suspension versus amoxicillin/clavulanate potassium, cefprozil, azithromycin, and amoxicillin in children aged 4 to 8 years. | Clinical therapeutics 20051201 |
| Effects of amoxicillin and cefdinir on nasopharyngeal bacterial flora. | Archives of otolaryngology--head & neck surgery 20050901 |
| A pooled comparison of cefdinir and penicillin in the treatment of group a beta-hemolytic streptococcal pharyngotonsillitis. | Clinical therapeutics 20050801 |
| Long-term effects on the nasopharyngeal flora of children following antimicrobial therapy of acute otitis media with cefdinir or amoxycillin-clavulanate. | Journal of medical microbiology 20050601 |
| A comparison of 5 days of therapy with cefdinir or azithromycin in children with acute otitis media: a multicenter, prospective, single-blind study. | Clinical therapeutics 20050601 |
| Cefdinir: an oral alternative to parenteral cephems. | Diagnostic microbiology and infectious disease 20050401 |
| [Effects of cefdinir in pediatric infectious diseases]. | Zhonghua er ke za zhi = Chinese journal of pediatrics 20050301 |
| Estimation of the intervention effect in a non-randomized study with pre- and post-mismeasured binary responses. | Statistics in medicine 20050215 |
| Cefdinir activity against contemporary North American isolates from community-acquired urinary tract infections. | International journal of antimicrobial agents 20050101 |
| Determination of Cefdinir by a stability-indicating liquid chromatographic method. | Journal of AOAC International 20050101 |
| Cefdinir versus levofloxacin in patients with acute rhinosinusitis of presumed bacterial etiology: a multicenter, randomized, double-blind study. | Clinical therapeutics 20041201 |
| [Comparison of pharmacokinetics/pharmacodynamics of cefdinir, cefpodoxime proxetil and cefaclor against common bacteria of community acquired infections]. | Zhonghua yi xue za zhi 20041117 |
| [Comparative study on cefdinir and cefaclor in the treatment of patients with mild to moderate bacterial community acquired pneumonia]. | Zhonghua yi xue za zhi 20041117 |
| Comparison of five-day cefdinir treatment with ten-day low dose amoxicillin/clavulanate treatment for acute otitis media. | The Pediatric infectious disease journal 20040901 |
| [In vitro antibacterial activity of cefdinir against isolates of respiratory tract pathogens in children]. | Zhonghua er ke za zhi = Chinese journal of pediatrics 20040901 |
| Fungal biofilm formation on cochlear implant hardware after antibiotic-induced fungal overgrowth within the middle ear. | The Pediatric infectious disease journal 20040801 |
| Potency and spectrum reevaluation of cefdinir tested against pathogens causing skin and soft tissue infections: a sample of North American isolates. | Diagnostic microbiology and infectious disease 20040801 |
| Overview of cefdinir: pharmacokinetics, safety, and efficacy in the treatment of uncomplicated skin and skin structure infections. | Cutis 20040501 |
| Effect of cefixime and cefdinir, oral cephalosporins, on cytochrome P450 activities in human hepatic microsomes. | Biological & pharmaceutical bulletin 20040101 |
| Cefdinir: a review of its use in the management of mild-to-moderate bacterial infections. | Drugs 20040101 |
| Parent-reported outcomes for treatment of acute otitis media with cefdinir or amoxicillin/clavulanate oral suspensions. | Paediatric drugs 20040101 |
| Contemporary evaluation of the in vitro activity and spectrum of cefdinir compared with other orally administered antimicrobials tested against common respiratory tract pathogens (2000-2002). | Diagnostic microbiology and infectious disease 20031101 |
| [Clinical efficacy and bacteriological studies of clarithromycin and cefdinir against group A beta-hemolytic streptococcal tonsillopharyngitis]. | The Japanese journal of antibiotics 20030801 |
| An alternative procedure for preparation of cefdinir. | Farmaco (Societa chimica italiana : 1989) 20030601 |
| [Susceptibility of major pathogens of acute pharyngitis and tonsillitis to levofloxacin and other oral antimicrobial drugs]. | The Japanese journal of antibiotics 20030601 |
| Management update of acute bacterial rhinosinusitis and the use of cefdinir. | Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 20021201 |
| [Antibacterial activity of gatifloxacin against various fresh clinical isolates in 2002]. | The Japanese journal of antibiotics 20021201 |
| [Antibacterial activity of cefpodoxime against clinical isolates in 2000 and 2001]. | The Japanese journal of antibiotics 20021201 |
| Cefdinir: an advanced-generation, broad-spectrum oral cephalosporin. | Clinical therapeutics 20020401 |
| Antibacterial action of several tannins against Staphylococcus aureus. | The Journal of antimicrobial chemotherapy 20011001 |
| Evaluation of oral antimicrobial agent levels in tooth extraction sites. | Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 20010601 |
| [Toxicity study of cefmatilen hydrochloride hydrate (S-1090) (5)--Six-month repeated oral dose toxicity study and supplement study in rats]. | The Journal of toxicological sciences 20010501 |
| Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. | Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy 20010301 |
| Successful steroid therapy for cefdinir-induced acute tubulointerstitial nephritis with progressive renal failure. | Internal medicine (Tokyo, Japan) 20010201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.