200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 17817-31-1
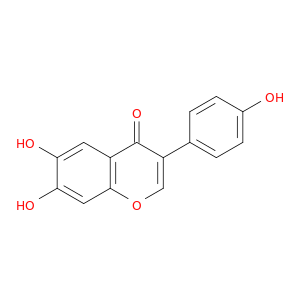
17817-31-1 | 4H-1-Benzopyran-4-one, 6,7-dihydroxy-3-(4-hydroxyphenyl)-
CAS No: 17817-31-1 Catalog No: AG0025TI MDL No:MFCD00016953
Product Description
Catalog Number:
AG0025TI
Chemical Name:
4H-1-Benzopyran-4-one, 6,7-dihydroxy-3-(4-hydroxyphenyl)-
CAS Number:
17817-31-1
Molecular Formula:
C15H10O5
Molecular Weight:
270.2369
MDL Number:
MFCD00016953
IUPAC Name:
6,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one
InChI:
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-14-6-13(18)12(17)5-10(14)15(11)19/h1-7,16-18H
InChI Key:
GYLUFQJZYAJQDI-UHFFFAOYSA-N
SMILES:
Oc1ccc(cc1)c1coc2c(c1=O)cc(c(c2)O)O
UNII:
PLM2K574GE
Properties
Complexity:
411
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
270.053g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
270.24g/mol
Monoisotopic Mass:
270.053g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
87A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.1
Literature
| Title | Journal |
|---|---|
| 6,7,4'-trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. | Carcinogenesis 20110401 |
| Chemometric modeling of free radical scavenging activity of flavone derivatives. | European journal of medicinal chemistry 20101101 |
| [Chemical constituents of bear bile]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20100901 |
| Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor alpha and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. | Menopause (New York, N.Y.) 20100301 |
| Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. | Nutrients 20100301 |
| Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. | Bioorganic & medicinal chemistry letters 20100201 |
| 7-Hydroxy-benzopyran-4-one derivatives: a novel pharmacophore of peroxisome proliferator-activated receptor alpha and -gamma (PPARalpha and gamma) dual agonists. | Journal of medicinal chemistry 20091112 |
| A-ring ortho-specific monohydroxylation of daidzein by cytochrome P450s of Nocardia farcinica IFM10152. | Biotechnology journal 20091101 |
| Inhibitory effect of flavonoids on 26S proteasome activity. | Journal of agricultural and food chemistry 20091028 |
| Isolation of a new metabolite from biotransformation of daidzein by Aspergillus oryzae. | Bioscience, biotechnology, and biochemistry 20090801 |
| An updated review of tyrosinase inhibitors. | International journal of molecular sciences 20090601 |
| Receptor binding and transactivation activities of red clover isoflavones and their metabolites. | The Journal of steroid biochemistry and molecular biology 20081101 |
| ortho-dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. | Bioorganic & medicinal chemistry letters 20080915 |
| Estrogenic activities of isoflavones and flavones and their structure-activity relationships. | Planta medica 20080101 |
| Red clover extract: a putative source for simultaneous treatment of menopausal disorders and the metabolic syndrome. | Menopause (New York, N.Y.) 20080101 |
| Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2. | Bioorganic & medicinal chemistry 20071201 |
| In vitro and in vivo metabolism of the soy isoflavone glycitein. | Molecular nutrition & food research 20070701 |
| Prediction of estrogen receptor agonists and characterization of associated molecular descriptors by statistical learning methods. | Journal of molecular graphics & modelling 20061101 |
| Metabolism of the soyabean isoflavone daidzein by CYP1A2 and the extra-hepatic CYPs 1A1 and 1B1 affects biological activity. | Biochemical pharmacology 20060828 |
| Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. | The Prostate 20060401 |
| Isoflavones modulate the glucuronidation of estradiol in human liver microsomes. | Carcinogenesis 20051201 |
| Identifying 6,7,4'-trihydroxyisoflavone as a potent tyrosinase inhibitor. | Bioscience, biotechnology, and biochemistry 20051001 |
| DPPH radical-scavenging compounds from dou-chi, a soybean fermented food. | Bioscience, biotechnology, and biochemistry 20050501 |
| Novel tempeh (fermented soyabean) isoflavones inhibit in vivo angiogenesis in the chicken chorioallantoic membrane assay. | The British journal of nutrition 20050301 |
| [Mechanism of mono-hydroxylation of daidzein in human liver microsomes]. | Yao xue xue bao = Acta pharmaceutica Sinica 20041101 |
| Isolation of 8-hydroxyglycitein and 6-hydroxydaidzein from soybean miso. | Bioscience, biotechnology, and biochemistry 20040601 |
| Genistein and daidzein induce cell proliferation and their metabolites cause oxidative DNA damage in relation to isoflavone-induced cancer of estrogen-sensitive organs. | Biochemistry 20040309 |
| Evidence for the involvement of human liver microsomes CYP1A2 in the mono-hydroxylation of daidzein. | Clinica chimica acta; international journal of clinical chemistry 20030801 |
| Antimutagenic activity of 8-hydroxyisoflavones and 6-hydroxydaidzein from soybean miso. | Bioscience, biotechnology, and biochemistry 20030401 |
| Fatty acid synthase inhibitors from plants: isolation, structure elucidation, and SAR studies. | Journal of natural products 20021201 |
| Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. | Journal of agricultural and food chemistry 20010601 |
| Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. | Chemical research in toxicology 20010301 |
| Flavonoid 6-hydroxylase from soybean (Glycine max L.), a novel plant P-450 monooxygenase. | The Journal of biological chemistry 20010119 |
Related Products
© 2019 Angene International Limited. All rights Reserved.