200,000+ products from a single source!
sales@angenechem.com
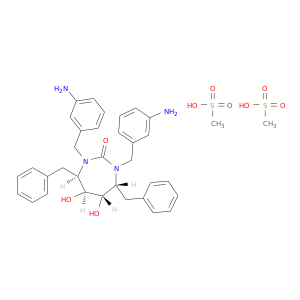
177932-89-7 | 2H-1,3-Diazepin-2-one, 1,3-bis[(3-aminophenyl)methyl]hexahydro-5,6-dihydroxy-4,7-bis(phenylmethyl)-, (4R,5S,6S,7R)-, methanesulfonate (1:2)
CAS No: 177932-89-7 Catalog No: AG0025M6 MDL No:
Product Description
Catalog Number:
AG0025M6
Chemical Name:
2H-1,3-Diazepin-2-one, 1,3-bis[(3-aminophenyl)methyl]hexahydro-5,6-dihydroxy-4,7-bis(phenylmethyl)-, (4R,5S,6S,7R)-, methanesulfonate (1:2)
CAS Number:
177932-89-7
Molecular Formula:
C35H44N4O9S2
Molecular Weight:
728.8753
IUPAC Name:
(4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-dibenzyl-5,6-dihydroxy-1,3-diazepan-2-one;methanesulfonic acid
InChI:
InChI=1S/C33H36N4O3.CH4O3S/c34-27-15-7-13-25(17-27)21-36-29(19-23-9-3-1-4-10-23)31(38)32(39)30(20-24-11-5-2-6-12-24)37(33(36)40)22-26-14-8-16-28(35)18-26;1-5(2,3)4/h1-18,29-32,38-39H,19-22,34-35H2;1H3,(H,2,3,4)/t29-,30-,31+,32+;/m1./s1
InChI Key:
RTAMCEYTLJCPQU-XXBGQOOISA-N
SMILES:
CS(=O)(=O)O.CS(=O)(=O)O.Nc1cccc(c1)CN1[C@H](Cc2ccccc2)[C@H](O)[C@H]([C@H](N(C1=O)Cc1cccc(c1)N)Cc1ccccc1)O
Properties
Complexity:
816
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
632.267g/mol
Formal Charge:
0
Heavy Atom Count:
45
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
632.776g/mol
Monoisotopic Mass:
632.267g/mol
Rotatable Bond Count:
8
Topological Polar Surface Area:
179A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Inhibitor docking screened by the modified SAFE_p scoring function: application to cyclic urea HIV-1 PR inhibitors. | Journal of computational chemistry 20071001 |
| Catalytic carbonylation of functionalized diamines: application to the core structure of DMP 323 and DMP 450. | The Journal of organic chemistry 20030221 |
| Nonsymmetric P2/P2' cyclic urea HIV protease inhibitors. Structure-activity relationship, bioavailability, and resistance profile of monoindazole-substituted P2 analogues. | Journal of medicinal chemistry 19980618 |
| Molecular recognition of cyclic urea HIV-1 protease inhibitors. | The Journal of biological chemistry 19980515 |
| The synthesis and evaluation of cyclic ureas as HIV protease inhibitors: modifications of the P1/P1' residues. | Bioorganic & medicinal chemistry letters 19980407 |
| Molecular basis of HIV-1 protease drug resistance: structural analysis of mutant proteases complexed with cyclic urea inhibitors. | Biochemistry 19970218 |
| Preparation and structure-activity relationship of novel P1/P1'-substituted cyclic urea-based human immunodeficiency virus type-1 protease inhibitors. | Journal of medicinal chemistry 19960524 |
| Improved cyclic urea inhibitors of the HIV-1 protease: synthesis, potency, resistance profile, human pharmacokinetics and X-ray crystal structure of DMP 450. | Chemistry & biology 19960401 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







