200,000+ products from a single source!
sales@angenechem.com
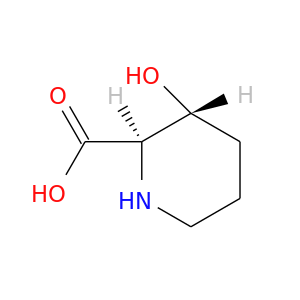
176019-04-8 | 2-Piperidinecarboxylic acid, 3-hydroxy-, (2R,3R)-
CAS No: 176019-04-8 Catalog No: AG0021DN MDL No:
Product Description
Catalog Number:
AG0021DN
Chemical Name:
2-Piperidinecarboxylic acid, 3-hydroxy-, (2R,3R)-
CAS Number:
176019-04-8
Molecular Formula:
C6H11NO3
Molecular Weight:
145.1564
IUPAC Name:
(2R,3R)-3-hydroxypiperidine-2-carboxylic acid
InChI:
InChI=1S/C6H11NO3/c8-4-2-1-3-7-5(4)6(9)10/h4-5,7-8H,1-3H2,(H,9,10)/t4-,5-/m1/s1
InChI Key:
FDMYUQHVJYNDLI-RFZPGFLSSA-N
SMILES:
O[C@@H]1CCCN[C@H]1C(=O)O
Properties
Complexity:
137
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
145.074g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
145.158g/mol
Monoisotopic Mass:
145.074g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
69.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.8
Literature
| Title | Journal |
|---|---|
| Synthesis of both enantiomers of hydroxypipecolic acid derivatives equivalent to 5-azapyranuronic acids and evaluation of their inhibitory activities against glycosidases. | Bioorganic & medicinal chemistry 20080901 |
| Synthesis of all stereoisomers of 3-hydroxypipecolic acid and 3-hydroxy-4,5-dehydropipecolic acid and their evaluation as glycosidase inhibitors. | Bioorganic & medicinal chemistry letters 20080315 |
| Stereoselective total synthesis of cis- and trans-3-hydroxypipecolic acid. | The Journal of organic chemistry 20051125 |
| Asymmetric synthesis of both the enantiomers of trans-3-hydroxypipecolic acid. | The Journal of organic chemistry 20050107 |
Related Products
© 2019 Angene International Limited. All rights Reserved.